Abstract
Purpose
Our study aimed to identify the genetic causes of non-syndromic primary ovarian insufficiency (POI) in female patients.
Methods
We performed whole exome sequencing in females suffering from isolated POI and in their available family members. Copy number variations were validated by long-range PCR and Sanger sequencing, and conservation analysis was used to evaluate the impact of sequence variants on protein composition.
Results
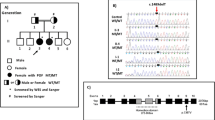
We detected two pathogenic TP63 heterozygous deleterious single nucleotide variants and a novel TP63 intragenic copy number alteration in three unrelated women with isolated POI. Two of these genetic variants are predicted to result in loss of transactivation inhibition of p63, whereas the third one affects the first exon of the ΔNp63 isoforms.
Conclusion
Our results broaden the spectrum of TP63-related disorders, which now includes sporadic and familial, isolated, and syndromic POI. Genomic variants that impair the transactivation inhibitory domain of the TAp63α isoform are the cause of non-syndromic POI. Additionally, variants affecting only the ΔNp63 isoforms may result in isolated POI. In patients with isolated POI, careful evaluation of genomic variants in pleiotropic genes such as TP63 will be essential to establish a full clinical spectrum and atypical presentation of a disorder.


Similar content being viewed by others
Data availability
Some or all datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.
References
Yatsenko SA, Rajkovic A. Genetics of human female infertility. Biol Reprod. 2019;101(3):549–66.
Titus S, et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med. 2013;5(172):172ra21.
Ruth KS, et al. Genetic insights into biological mechanisms governing human ovarian ageing. Nature. 2021;596(7872):393–7.
Osterburg C, Osterburg S, Zhou H, Missero C, Dötsch V. Isoform-specific roles of mutant p63 in human diseases. Cancers. 2021;13(3)3):536. https://doi.org/10.3390/cancers13030536.
Osterburg C, Dotsch V. Structural diversity of p63 and p73 isoforms. Cell Death Differ. 2022;29(5):921–37.
Deutsch GB, et al. DNA damage in oocytes induces a switch of the quality control factor TAp63alpha from dimer to tetramer. Cell. 2011;144(4):566–76.
Coutandin D, Osterburg C, Srivastav RK, Sumyk M, Kehrloesser S, Gebel J, Tuppi M, Hannewald J, Schäfer B, Salah E, Mathea S, Müller-Kuller U, Doutch J, Grez M, Knapp S, Dötsch V. Quality control in oocytes by p63 is based on a spring-loaded activation mechanism on the molecular and cellular level. Elife. 2016;5:e13909. https://doi.org/10.7554/eLife.13909.
Senoo M, et al. p63 Is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129(3):523–36.
Yang A, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398(6729):714–8.
Suh EK, et al. p63 protects the female germ line during meiotic arrest. Nature. 2006;444(7119):624–8.
Tuppi M, et al. Oocyte DNA damage quality control requires consecutive interplay of CHK2 and CK1 to activate p63. Nat Struct Mol Biol. 2018;25(3):261–9.
Gonfloni S, et al. Inhibition of the c-Abl-TAp63 pathway protects mouse oocytes from chemotherapy-induced death. Nat Med. 2009;15(10):1179–85.
Bolcun-Filas E, et al. Reversal of female infertility by Chk2 ablation reveals the oocyte DNA damage checkpoint pathway. Science. 2014;343(6170):533–6.
Lena AM, et al. The p63 C-terminus is essential for murine oocyte integrity. Nat Commun. 2021;12(1):383.
Sutton VR, van Bokhoven H. TP63-related disorders. 2010 Jun 8 [updated 2021 Apr 1]. In: Adam MP, Mirzaa GM, Pagon RA, Wallace SE, Bean LJH, Gripp KW, Amemiya A, editors. GeneReviews® [Internet]. University of Washington, Seattle; 1993–2023.
Amiel J, et al. TP63 gene mutation in ADULT syndrome. Eur J Hum Genet. 2001;9(8):642–5.
Holder-Espinasse M, et al. A new mutation in TP63 is associated with age-related pathology. Eur J Hum Genet. 2007;15(11):1115–20.
Guazzarotti L, et al. Limb-mammary syndrome (LMS) associated with internal female genitalia dysgenesia: a new genotype/phenotype correlation? Am J Med Genet A. 2008;146A(15):2001–4.
Mathorne SW, et al. Novel phenotype of syndromic premature ovarian insufficiency associated with TP63 molecular defect. Clin Genet. 2020;97(5):779–84.
Bestetti I, et al. High-resolution array-CGH analysis on 46,XX patients affected by early onset primary ovarian insufficiency discloses new genes involved in ovarian function. Hum Reprod. 2019;34(3):574–83.
Tucker EJ, et al. TP63-truncating variants cause isolated premature ovarian insufficiency. Hum Mutat. 2019;40(7):886–92.
Tucker EJ, et al. Dominant TP63 missense variants lead to constitutive activation and premature ovarian insufficiency. Hum Mutat. 2022;43(10):1443–53.
Yatsenko SA, et al. Pathogenic variants in ZSWIM7 cause primary ovarian insufficiency. J Clin Endocrinol Metab. 2022;107(6):e2359–64.
Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38(16):e164.
McWilliam H, et al. Analysis tool web services from the EMBL-EBI. Nucleic Acids Res. 2013;41(Web Server issue):W597–600.
Russo C, et al. Protein aggregation of the p63 transcription factor underlies severe skin fragility in AEC syndrome. Proc Natl Acad Sci U S A. 2018;115(5):E906–15.
Helton ES, Zhu J, Chen X. The unique NH2-terminally deleted (DeltaN) residues, the PXXP motif, and the PPXY motif are required for the transcriptional activity of the DeltaN variant of p63. J Biol Chem. 2006;281(5):2533–42.
Craig AL, et al. DeltaNp63 transcriptionally regulates ATM to control p53 Serine-15 phosphorylation. Mol Cancer. 2010;9:195.
Chan WM, et al. How many mutant p53 molecules are needed to inactivate a tetramer? Mol Cell Biol. 2004;24(8):3536–51.
Westfall MD, et al. The Delta Np63 alpha phosphoprotein binds the p21 and 14-3-3 sigma promoters in vivo and has transcriptional repressor activity that is reduced by Hay-Wells syndrome-derived mutations. Mol Cell Biol. 2003;23(7):2264–76.
Yang A, et al. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2(3):305–16.
Onn L, Portillo M, Ilic S, Cleitman G, Stein D, Kaluski S, Shirat I, Slobodnik Z, Einav M, Erdel F, Akabayov B, Toiber D. SIRT6 is a DNA double-strand break sensor. Elife. 2020:9:e51636. https://doi.org/10.7554/eLife.51636.
van Bokhoven H, et al. p63 Gene mutations in eec syndrome, limb-mammary syndrome, and isolated split hand-split foot malformation suggest a genotype-phenotype correlation. Am J Hum Genet. 2001;69(3):481–92.
Ianakiev P, et al. Split-hand/split-foot malformation is caused by mutations in the p63 gene on 3q27. Am J Hum Genet. 2000;67(1):59–66.
Khandelwal KD, et al. Deletions and loss-of-function variants in TP63 associated with orofacial clefting. Eur J Hum Genet. 2019;27(7):1101–12.
Wenger T, et al. Expanding the phenotypic spectrum of TP63-related disorders including the first set of monozygotic twins. Am J Med Genet A. 2018;176(1):75–81.
Marcet-Ortega M, et al. p53 and TAp63 participate in the recombination-dependent pachytene arrest in mouse spermatocytes. PLoS Genet. 2017;13(6):e1006845.
Gebel J, et al. DNA damaged induced cell death in oocytes. Molecules. 2020;25(23). https://doi.org/10.3390/molecules25235714
Luan Y, et al. TAp63 determines the fate of oocytes against DNA damage. Sci Adv. 2022;8(51):eade1846.
Acknowledgements
We are grateful to the patients and their families for participating in this study as well as Jessica Settino for a technical assistance.
Funding
This work was supported by the National Institute of Child Health and Human Development (R01HD070647 and R21HD074278, to AR) and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (5T32HD007263-38 to MR-E).
Author information
Authors and Affiliations
Contributions
SHC, SAY, and AR collected the clinical information; SAY and AR conceived the experiments; RKV, MRE, SAY, and AR analyzed experimental data; MRE and AJB performed conservation analysis and in silico modeling. The manuscript was written by RKV, MRE, and SAY; reviewed by AJB, SHC, and AR. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The authors consider that the first two authors should be regarded as joint First Authors
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vanderschelden, R.K., Rodriguez-Escriba, M., Chan, S.H. et al. Heterozygous TP63 pathogenic variants in isolated primary ovarian insufficiency. J Assist Reprod Genet 40, 2211–2218 (2023). https://doi.org/10.1007/s10815-023-02886-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-023-02886-w