Abstract
Purpose
To investigate whether embryo rebiopsy increases the yield of in vitro fertilization (IVF) cycles.
Methods
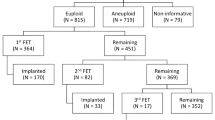
Retrospective study including 18,028 blastocysts submitted for trophectoderm biopsy and preimplantation genetic testing for aneuploidy (PGT-A) between January 2016 and December 2021 in a private IVF center. Out of the 517 embryos categorized as inconclusive, 400 survived intact to the warming procedure, re-expanded, and were suitable for rebiopsy. Of them, 71 rebiopsied blastocysts were transferred. Factors affecting the probability of obtaining an undiagnosed blastocyst and clinical outcomes from blastocysts biopsied once and twice were investigated.
Results
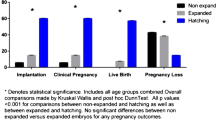
The overall diagnostic rate was 97.1%, with 517 blastocysts receiving inconclusive reports. Several blastocyst and laboratory features, such as the day of the biopsy, the stage of development, and the biopsy methodology, were related to the risk of obtaining an inconclusive diagnosis after PGT-A. A successful diagnosis was obtained in 384 of the rebiopsied blastocysts, 238 of which were chromosomally transferable. A total of 71 rebiopsied blastocysts were transferred, resulting in 32 clinical pregnancies [(clinical pregnancy rate (CPR)=45.1%], 16 miscarriages [(miscarriage rate (MR)=41%], and, until September 2020, 12 live births [(live birth rate (LBR)=23.1%]. A significantly lower LBR and higher MR were obtained after transferring rebiopsied blastocysts compared to those biopsied once.
Conclusion
Although an extra round of biopsy and vitrification may cause a detrimental effect on embryo viability, re-analyzing the test-failure blastocysts contributes to increasing the number of euploid blastocysts available for transfer and the LBR.

Similar content being viewed by others
Data availability
Data regarding any of the subjects in the study have not been previously published.
References
Van Montfoort A, De Rycke M, Carvalho F, Rubio C, Bronet F, Spinella F, Goossens V. O-041 Data from the ESHRE PGT consortium – year 2020. Hum Reprod. 2022;37:deac104–047. https://doi.org/10.1093/humrep/deac104.047.
Maheshwari A, Raja EA, Bhattacharya S. Obstetric and perinatal outcomes after either fresh or thawed frozen embryo transfer: an analysis of 112,432 singleton pregnancies recorded in the Human Fertilisation and Embryology Authority anonymized dataset. Fertil Steril. 2016;106:1703–8. https://doi.org/10.1016/j.fertnstert.2016.08.047.
Roy TK, Bradley CK, Bowman MC, SJ MA. Single-embryo transfer of vitrified-warmed blastocysts yields equivalent live-birth rates and improved neonatal outcomes compared with fresh transfers. Fertil Steril. 2014;101:1294–301. https://doi.org/10.1016/j.fertnstert.2014.01.046.
Kato O, Kawasaki N, Bodri D, Kuroda T, Kawachiya S, Kato K, Takehara Y. Neonatal outcome and birth defects in 6623 singletons born following minimal ovarian stimulation and vitrified versus fresh single embryo transfer. Eur J Obstet Gynecol Reprod Biol. 2012;161:46–50. https://doi.org/10.1016/j.ejogrb.2011.12.005.
Li X, Li W, Jia H, Gao Y, Shi W, Bai H. Double vitrification-warming cycles, coupled with blastocyst biopsy, impair live birth but do not affect neonatal outcomes. Int J Gynaecol Obstet. 2022;160(3):806–13. https://doi.org/10.1002/ijgo.14355.
Wang M, Jiang J, Xi Q, Li D, Ren X, Li Z, Zhu L, Jin L. Repeated cryopreservation process impairs embryo implantation potential but does not affect neonatal outcomes. Reprod Biomed Online. 2021;42:75–82. https://doi.org/10.1016/j.rbmo.2020.11.007.
Cobo A, Castello D, Vallejo B, Albert C, de los Santos JM, Remohi J. Outcome of cryotransfer of embryos developed from vitrified oocytes: double vitrification has no impact on delivery rates. Fertil Steril. 2013;99:1623–30. https://doi.org/10.1016/j.fertnstert.2013.01.106.
Zhang S, Luo K, Cheng D, Tan Y, Lu C, He H, Gu Y, Lu G, Gong F, Lin G. Number of biopsied trophectoderm cells is likely to affect the implantation potential of blastocysts with poor trophectoderm quality. Fertil Steril. 2016;105:1222–1227.e4. https://doi.org/10.1016/j.fertnstert.2016.01.011.
Neal SA, Morin SJ, Tiegs AW, Sun L, Franasiak JM, Kaser DJ, Hong KH, Werner MD, Scott RT Jr. Repeat biopsy for preimplantation genetic screening (PGS) reanalysis does not adversely impact obstetrical outcomes. Fertil Steril. 2018;109:e41. https://doi.org/10.1016/j.fertnstert.2018.02.080.
Lee H, McCulloh DH, Olivares R, Goldstein-Tufaro A, McCaffrey C, Grifo J. Live births after transfer of rebiopsy and revitrification of blastocyst that had “no diagnosis” following trophectoderm biopsy. Fertil Steril. 2016;106:e164. https://doi.org/10.1016/j.fertnstert.2016.07.483.
Kaing A, Kroener L, Brower M, Hill D, Danzer H, Barritt J. Rebiopsy and preimplanation genetic screening (PGS) reanalysis demonstrate the majority of originally “no diagnosis” embryos are euploid with comparable pregnancy rates. Fertil Steril. 2015;104:e277. https://doi.org/10.1016/j.fertnstert.2015.07.869.
Swain JE, Schoolcraft WB, Katz-Jaffe M. Dual trophectoderm biopsy on the same blastocyst does not impair clinical outcomes. Fertil Steril. 2015;104:e186. https://doi.org/10.1016/j.fertnstert.2015.07.577.
Brower M, Hill D, Danzer H, Surrey M, Ghadir S, Chang W, Wambach C, Alexander C, Barritt J. “No diagnosis” embryos after PGS should not be discarded: rebiopsy and reanalysis demonstrate the majority are euploid. Fertil Steril. 2014;102:e31. https://doi.org/10.1016/j.fertnstert.2014.07.114.
De Vos A, Van Landuyt L, De Rycke M, Verdyck P, Verheyen G, Buysse A, Belva F, Keymolen K, Tournaye H, Verpoest W. Multiple vitrification-warming and biopsy procedures on human embryos: clinical outcome and neonatal follow-up of children. Hum Reprod. 2020;35:2488–96. https://doi.org/10.1093/humrep/deaa236.
Neal SA, Sun L, Jalas C, Morin SJ, Molinaro TA, RTJ S. When next-generation sequencing-based preimplantation genetic testing for aneuploidy (PGT-A) yields an inconclusive report: diagnostic results and clinical outcomes after re biopsy. J Assist Reprod Genet. 2019;36:2103–9. https://doi.org/10.1007/s10815-019-01550-6.
Parriego M, Coll L, Vidal F, Boada M, Devesa M, Coroleu B, Veiga A. Inconclusive results in preimplantation genetic testing: go for a second biopsy? Gynecol Endocrinol. 2019;35:90–2. https://doi.org/10.1080/09513590.2018.1497153.
Cimadomo D, Rienzi L, Romanelli V, Alviggi E, Levi-Setti PE, Albani E, Dusi L, Papini L, Livi C, Benini F, Smeraldi A, Patassini C, Ubaldi FM, Capalbo A. Inconclusive chromosomal assessment after blastocyst biopsy: prevalence, causative factors and outcomes after re-biopsy and re-vitrification. A multicenter experience. Hum Reprod. 2018;33:1839–46. https://doi.org/10.1093/humrep/dey282.
Bradley CK, Livingstone M, Traversa MV, SJ MA. Impact of multiple blastocyst biopsy and vitrification-warming procedures on pregnancy outcomes. Fertil Steril. 2017;108:999–1006. https://doi.org/10.1016/j.fertnstert.2017.09.013.
Zhang S, Tan K, Gong F, Gu Y, Tan Y, Lu C, Luo K, Lu G, Lin G. Blastocysts can be rebiopsied for preimplantation genetic diagnosis and screening. Fertil Steril. 2014;102:1641–5. https://doi.org/10.1016/j.fertnstert.2014.09.018.
Bori L, Meseguer F, Valera MA, Galan A, Remohi J, Meseguer M. The higher the score, the better the clinical outcome: retrospective evaluation of automatic embryo grading as a support tool for embryo selection in IVF laboratories. Hum Reprod. 2022;37:1148–60. https://doi.org/10.1093/humrep/deac066.
Ardoy M, Calderón G, Arroyo G, Cuadros J, Figueroa M, Herrer R. ASEBIR criteria for the morphological evaluation of human oocytes, early embryos and blastocysts. In: ASEBIR clinical embryology papers; 2008.
Gardner DK. Schoolcraft WB In vitro culture of human blastocysts: towards reproductive certainty: infertility and genetics beyond. Carnforth: Parthenon Press; 1999.
Boada M, Carrera M, De La Iglesia C, Sandalinas M, Barri PN, Veiga A. Successful use of a laser for human embryo biopsy in preimplantation genetic diagnosis: report of two cases. J Assist Reprod Genet. 1998;15:302–7. https://doi.org/10.1023/a:1022548612107.
Coll L, Parriego M, Carrasco B, Rodriguez I, Boada M, Coroleu B, Polyzos NP, Vidal F, Veiga A. The effect of trophectoderm biopsy technique and sample handling on artefactual mosaicism. J Assist Reprod Genet. 2022;39:1333–40. https://doi.org/10.1007/s10815-022-02453-9.
Mizobe Y, Kuwatsuru Y, Kuroki Y, Fukumoto Y, Tokudome M, Moewaki H, Watanabe M, Iwakawa T, Takeuchi K. The effects of differences in trophectoderm biopsy techniques and the number of cells collected for biopsy on next-generation sequencing results. Reprod Med Biol. 2022;21:e12463. https://doi.org/10.1002/rmb2.12463.
Martin A, Rodrigo L, Beltran D, Meseguer M, Rubio C, Mercader A, de Los Santos MJ. The morphokinetic signature of mosaic embryos: evidence in support of their own genetic identity. Fertil Steril. 2021;116:165–73. https://doi.org/10.1016/j.fertnstert.2020.12.031.
Cobo A, Vajta G, Remohi J. Vitrification of human mature oocytes in clinical practice. Reprod Biomed Online. 2009;19(Suppl 4):4385.
Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online. 2005;11:300–8. https://doi.org/10.1016/s1472-6483(10)60837-1.
Tiegs AW, Tao X, Zhan Y, Whitehead C, Kim J, Hanson B, Osman E, Kim TJ, Patounakis G, Gutmann J, Castelbaum A, Seli E, Jalas C, RTJ S. A multicenter, prospective, blinded, nonselection study evaluating the predictive value of an aneuploid diagnosis using a targeted next-generation sequencing-based preimplantation genetic testing for aneuploidy assay and impact of biopsy. Fertil Steril. 2021;115:627–37. https://doi.org/10.1016/j.fertnstert.2020.07.052.
Coello A, Nohales M, Meseguer M, de Los Santos MJ, Remohi J, Cobo A. Prediction of embryo survival and live birth rates after cryotransfers of vitrified blastocysts. Reprod Biomed Online. 2021;42:881–91. https://doi.org/10.1016/j.rbmo.2021.02.013.
Schoolcraft WB, Treff NR, Stevens JM, Ferry K, Katz-Jaffe M, RTJ S. Live birth outcome with trophectoderm biopsy, blastocyst vitrification, and single-nucleotide polymorphism microarray-based comprehensive chromosome screening in infertile patients. Fertil Steril. 2011;96:638–40. https://doi.org/10.1016/j.fertnstert.2011.06.049.
Cram DS, Leigh D, Handyside A, Rechitsky L, Xu K, Harton G, Grif RC, Fragouli E, Kahraman S, Forman E, Katz-Jaffe M, Tempest H, Thornhill A, Strom C, Escudero T, Qiao J, Munne S, Simpson JL, Kuliev A. PGDIS Position Statement on the Transfer of Mosaic Embryos 2019. Reprod Biomed Online. 2019;39(Suppl 1):e1–4. https://doi.org/10.1016/j.rbmo.2019.06.012.
Kelk DA, Sawarkar SS, Liu Y, Dufton M, Ribustello L, Munne S. Does laser assisted biopsy introduce mosaic or chaotic changes to biopsied cells? Fertil Steril. 2017;108:e88. https://doi.org/10.1016/j.fertnstert.2017.07.272.
Johnson D, Haimowitz Z, Arifova M, Akopians A, Welch C, Barritt J. Repeated high intensity laser biopsy pulses do not alter genetic testing results nor increases mosaicism following human embryo blastocyst biopsy of trophectoderm cells. Reprod Biomed. 2019;39:e4–5. https://doi.org/10.1016/j.rbmo.2019.07.013.
Benavent M, Escriba M, Miret C, Vanrell I, Costa-Borges N, Calderón G, Crespo J, Teruel J. Evaluation of the impact of the pulling and flicking trophectoderm biopsy procedures on the integrity of the biopsied cells and their correlation to PGT-A results. Fertil Steril. 2019;112:e242. https://doi.org/10.1016/j.fertnstert.2019.07.1377.
Taylor TH, Patrick JL, Gitlin SA, Michael Wilson J, Crain JL, Griffin DK. Outcomes of blastocysts biopsied and vitrified once versus those cryopreserved twice for euploid blastocyst transfer. Reprod Biomed Online. 2014;29:59–64. https://doi.org/10.1016/j.rbmo.2014.03.001.
Alfarawati S, Fragouli E, Colls P, Stevens J, Gutierrez-Mateo C, Schoolcraft WB, Katz-Jaffe MG, Wells D. The relationship between blastocyst morphology, chromosomal abnormality, and embryo gender. Fertil Steril. 2011;95:520–4. https://doi.org/10.1016/j.fertnstert.2010.04.003.
Acknowledgements
The authors thank all the embryologists and technicians of the IVF laboratory at IVI-RMA Valencia (Spain), especially the biopsy team members, for their clinical and technical support in this study.
Author information
Authors and Affiliations
Contributions
All authors have materially participated in the research and/or article preparation. (1) M. Nohales, A. Coello, A. Martin, F. Insua, and MJ. De los Santos: the conception and design of the study, or acquisition of data, or analysis and interpretation of data. (2) M. Nohales, A. Martin, and M. Meseguer: drafting the article or revising it critically for important intellectual content. (3) A. Martin and M. Nohales: final approval of the version to be submitted.
Corresponding author
Ethics declarations
Ethics approval
All procedures were approved by our institutional review board (#2202-VLC-010-MN), the ethics committee of Clinical Research IVI-RMA Valencia, which regulates and approves the analysis of databases for research purposes. The project complies with the Spanish law governing assisted reproductive technologies (14/2006).
Consent to participate
Given its retrospective nature, formal consent of study participants was not required.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1:
Supplemental Table S1. Incidence of inconclusive results after PGT-A and frequency of technique usage across biopsy operators. Column proportions were compared using a Chi-square test with Bonferroni correction at the 95% confidence level. The same letters for each parameter indicate homogeneous subsets. PC: pairwise comparisons. PGT-A: Preimplantation Genetic Testing for Aneuploidy.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nohales, M., Coello, A., Martin, A. et al. Should embryo rebiopsy be considered a regular strategy to increase the number of embryos available for transfer?. J Assist Reprod Genet 40, 1905–1913 (2023). https://doi.org/10.1007/s10815-023-02875-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-023-02875-z