Abstract
Objective
To evaluate the distribution of chromosomal abnormalities in a recurrent pregnancy loss (RPL) cohort and explore the associations between chromosomal abnormalities and clinical characteristics.
Method
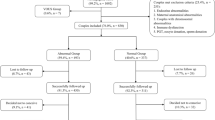
Over a 5-year period, fresh products of conception (POC) from women with RPL were analyzed by single-nucleotide polymorphism (SNP) array at our hospital. After obtaining the information on clinical characteristics, we investigated the associations between the causative chromosomal abnormalities and clinical characteristics by the chi-squared test or Fisher’s exact test and logistic regression.
Results
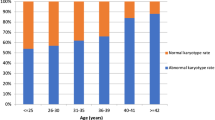
A total of 2383 cases were enrolled. Overall, 56.9% (1355/2383) were identified with causative chromosomal abnormalities, of which 92.1% (1248/1355) were numerical abnormalities, 7.5% (102/1355) were structural variants, and 0.4% (5/1355) were loss of heterozygosity (LOH). The risk of numerical abnormalities was increased in women with maternal age ≥ 35 years (OR, 1.71; 95% CI, 1.41–2.07), gestational age at pregnancy loss ≤ 12 weeks (OR, 2.78; 95% CI, 1.79–4.33), less number of previous pregnancy losses (twice: OR, 2.32; 95% CI, 1.84–2.94; 3 times: OR, 1.59; 95% CI, 1.23–2.05, respectively), and pregnancy with a female fetus (OR, 1.37; 95% CI, 1.15–1.62). The OR of pregnancy loss with recurrent abnormal CMA was 4.00 (95% CI: 1.87–8.58, P < 0.001) and the adjusted OR was 5.05 (95% CI: 2.00–12.72, P = 0.001). However, the mode of conception was not associated with the incidence of numerical abnormality. No association was noted between structural variants and clinical characteristics.
Conclusion
Chromosomal abnormality was the leading cause of RPL. Numerical chromosome abnormality was more likely to occur in cases with advanced maternal age, an earlier gestational age, fewer previous pregnancy losses, and pregnancy with a female fetus.


Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Practice Committee of the American Society for Reproductive M. Evaluation and treatment of recurrent pregnancy loss: a committee opinion. Fertil Steril. 2012;98(5):1103–11.
Bender Atik R, Christiansen OB, RPL EGGo, et al. ESHRE guideline: recurrent pregnancy loss. Hum Reprod Open. 2018;2018(2):4.
Rai R, Regan L. Recurrent miscarriage. The Lancet. 2006;368(9535):601–11.
Grande M, Borrell A, Garcia-Posada R, et al. The effect of maternal age on chromosomal anomaly rate and spectrum in recurrent miscarriage. Hum Reprod. 2012;27(10):3109–17.
Sullivan AE, Silver RM, LaCoursiere DY, Porter TF, Branch DW. Recurrent fetal aneuploidy and recurrent miscarriage. Obstet Gynecol. 2004;104(4):784–8.
Sheng YR, Hou SY, Hu WT, et al. Characterization of copy-number variations and possible candidate genes in recurrent pregnancy losses. Genes (Basel). 2021;12(2):141.
Ogasawara M, Aoki K, Okada S, Suzumori K. Embryonic karyotype of abortuses in relation to the number of previous miscarriages. Fertil Steril. 2000;73(2):300–4.
Gu C, Li K, Li R, et al. Chromosomal aneuploidy associated with clinical characteristics of pregnancy loss. Front Genet. 2021;12:667697.
Goldstein M, Svirsky R, Reches A, Yaron Y. Does the number of previous miscarriages influence the incidence of chromosomal aberrations in spontaneous pregnancy loss? J Matern Fetal Neonatal Med. 2017;30(24):2956–60.
Nikitina TV, Sazhenova EA, Zhigalina DI, Tolmacheva EN, Sukhanova NN, Lebedev IN. Karyotype evaluation of repeated abortions in primary and secondary recurrent pregnancy loss. J Assist Reprod Genet. 2020;37(3):517–25.
Hardy K, Hardy PJ, Jacobs PA, Lewallen K, Hassold TJ. Temporal changes in chromosome abnormalities in human spontaneous abortions: Results of 40 years of analysis. Am J Med Genet A. 2016;170(10):2671–80.
Qu S, Wang L, Cai A, et al. Exploring the cause of early miscarriage with SNP-array analysis and karyotyping. J Matern Fetal Neonatal Med. 2019;32(1):1–10.
Munne S, Sandalinas M, Magli C, Gianaroli L, Cohen J, Warburton D. Increased rate of aneuploid embryos in young women with previous aneuploid conceptions. Prenat Diagn. 2004;24(8):638–43.
Ogasawara M, Aoki K, Okada S, Suzumori K. Embryonic karyotype of abortuses in relation to the number of previous miscarriages. Fertil Steril. 2000;73(2):300–4.
Ma S, Philipp T, Zhao Y, Stetten G, Robinson WP, Kalousek D. Frequency of chromosomal abnormalities in spontaneous abortions derived from intracytoplasmic sperm injection compared with those from in vitro fertilization. Fertil Steril. 2006;85(1):236–9.
Lathi RB, Milki AA. Rate of aneuploidy in miscarriages following in vitro fertilization and intracytoplasmic sperm injection. Fertil Steril. 2004;81(5):1270–2.
Causio F, Fischetto R, Sarcina E, Geusa S, Tartagni M. Chromosome analysis of spontaneous abortions after in vitro fertilization (IVF) and intracytoplasmic sperm injection (ICSI). Europ J Obstet Gynecol Reprod Biol. 2002;105(1):44–8.
Sahoo T, Dzidic N, Strecker MN, et al. Comprehensive genetic analysis of pregnancy loss by chromosomal microarrays: outcomes, benefits, and challenges. Genet Med. 2017;19(1):83–9.
Wang Y, Li Y, Chen Y, et al. Systematic analysis of copy-number variations associated with early pregnancy loss. Ultrasound Obstet Gynecol. 2020;55(1):96–104.
Riggs ER, Andersen EF, Cherry AM, et al. Technical standards for the interpretation and reporting of constitutional copy-number variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen). Genet Med. 2020;22(2):245–57.
Tartaglia NR, Howell S, Sutherland A, Wilson R, Wilson L. A review of trisomy X (47, XXX). Orphanet J Rare Dis. 2010;5:8.
Bardsley MZ, Kowal K, Levy C, et al. 47, XYY syndrome: clinical phenotype and timing of ascertainment. J Pediatr. 2013;163(4):1085–94.
Hassold TJ, Jacobs PA. Trisomy in man. Annu Rev Genet. 1984;18:69–97.
Gruhn JR, Zielinska AP, Shukla V, et al. Chromosome errors in human eggs shape natural fertility over reproductive life span. Science. 2019;365(6460):1466–9.
Gardner RJM, Amor DJ. Gardner and Sutherland’s chromosome abnormalities and genetic counseling. 5th ed. Oxford University Press; 2018. p. 781.
Qiao Y, Wen J, Tang F, et al. Whole exome sequencing in recurrent early pregnancy loss. Mol Hum Reprod. 2016;22(5):364–72.
Zhao C, Chai H, Zhou Q, et al. Exome sequencing analysis on products of conception: a cohort study to evaluate clinical utility and genetic etiology for pregnancy loss. Genet Med. 2021;23(3):435–42.
Gourhant L, Bocher O, De Saint ML, et al. Whole exome sequencing, a hypothesis-free approach to investigate recurrent early miscarriage. Reprod Biomed Online. 2021;42(4):789–98.
Del Fabro A, Driul L, Anis O, et al. Fetal gender ratio in recurrent miscarriages. Int J Womens Health. 2011;3:213–7.
Cheng HH, Ou CY, Tsai CC, et al. Chromosome distribution of early miscarriages with present or absent embryos: female predominance. J Assist Reprod Genet. 2014;31(8):1059–64.
Patten MM. The X chromosome favors males under sexually antagonistic selection. Evolution. 2019;73(1):84–91.
Evdokimova VN, Nikitina TV, Lebedev IN, Sukhanova NN, Nazarenko SA. Sex ratio in early embryonal mortality in man. Ontogenez. 2000;31(4):251–7.
Sugiura-Ogasawara M, Ozaki Y, Katano K, Suzumori N, Kitaori T, Mizutani E. Abnormal embryonic karyotype is the most frequent cause of recurrent miscarriage. Hum Reprod. 2012;27(8):2297–303.
Finley J, Hay S, Oldzej J, et al. The genomic basis of sporadic and recurrent pregnancy loss: a comprehensive in-depth analysis of 24,900 miscarriages. Reprod Biomed Online. 2022;45(1):125–34.
Sangha KK, Stephenson MD, Brown CJ, Robinson WP. Extremely skewed X-chromosome inactivation is increased in women with recurrent spontaneous abortion. Am J Hum Genet. 1999;65(3):913–7.
Beever CL, Stephenson MD, Penaherrera MS, et al. Skewed X-chromosome inactivation is associated with trisomy in women ascertained on the basis of recurrent spontaneous abortion or chromosomally abnormal pregnancies. Am J Hum Genet. 2003;72(2):399–407.
Kim SY, Park SY, Choi JW, et al. Association between MTHFR 1298A>C polymorphism and spontaneous abortion with fetal chromosomal aneuploidy. Am J Reprod Immunol. 2011;66(4):252–8.
Wang Y, Cheng Q, Meng L, et al. Clinical application of SNP array analysis in first-trimester pregnancy loss: a prospective study. Clin Genet. 2017;91(6):849–58.
Fan L, Wu J, Wu Y, et al. Analysis of chromosomal copy number in first-trimester pregnancy loss using next-generation sequencing. Front Genet. 2020;11:545856.
Belva F, Bonduelle M, Buysse A, et al. Chromosomal abnormalities after ICSI in relation to semen parameters: results in 1114 fetuses and 1391 neonates from a single center. Hum Reprod. 2020;35(9):2149–62.
Samli H, Solak M, Imirzalioglu N, Beyatli Y, Simsek S, Kahraman S. Fetal chromosomal analysis of pregnancies following intracytoplasmic sperm injection with amniotic tissue culture. Prenat Diagn. 2003;23(10):847–50.
Kim JW, Lee WS, Yoon TK, et al. Chromosomal abnormalities in spontaneous abortion after assisted reproductive treatment. BMC Med Genet. 2010;11:153.
Orzack SH, Stubblefield JW, Akmaev VR, et al. The human sex ratio from conception to birth. Proc Natl Acad Sci U S A. 2015;112(16):E2102–11.
Liang D, Wang Y, Ji X, et al. Clinical application of whole-genome low-coverage next-generation sequencing to detect and characterize balanced chromosomal translocations. Clin Genet. 2017;91(4):605–10.
Chen Y, Bartanus J, Liang D, et al. Characterization of chromosomal abnormalities in pregnancy losses reveals critical genes and loci for human early development. Hum Mutat. 2017;38(6):669–77.
Acknowledgements
We thank all patients in our study, physicians who recorded the clinical data, and center members who supported us.
Funding
The study was supported by the National Key Research and Development Program of China (2022YFC2704700, 2022YFC2704703).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
The study was reviewed and approved by the Ethical Committee of Shanghai First Maternity and Infant Hospital (KS2203).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, D., Wei, X., Zhou, Xy. et al. Chromosomal abnormalities in recurrent pregnancy loss and its association with clinical characteristics. J Assist Reprod Genet 40, 1713–1720 (2023). https://doi.org/10.1007/s10815-023-02816-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-023-02816-w