Abstract
Objective
This study aims to evaluate the correlation combined fetal fraction and Z-score for fetal trisomies 13, 18, and 21 of NIPT by the semiconductor sequencing platform and further analyze the differences of different sequencing depths.
Methods
A cohort of 61,581 pregnancies were recruited for NIPT. Invasive prenatal diagnostic confirmation is recommended in all high-risk NIPT cases. Logistic regression and rank correlation analysis were applied to analyze the relationship between different parameters. ROC curve analysis was adopted to analyze the cutoff values of Z-score and fetal fraction.
Results
A total of 278 common trisomy pregnancies were verified in 377 NIPT-positive results. The fitted logistic regression models revealed that Z-scores of NIPT-positive results were significantly associated with PPVs (p < 0.05). The ROC curve analysis showed that the optimal cutoff value of Z-scores for T21, T18, and T13 was 7.597, 4.944, and 9.135 for NIPT and 9.489, 8.004, and 12.4 for NIPT-plus. If combing fetal fraction as another evaluation factor, the PPV of trisomy 21 gradually improved. We analyzed the correlation between the fetal fraction and the PPV, which revealed that the fetal fraction was significantly correlated with PPV. By analyzing the PPV of different groups divided by the associated criteria obtained from ROC curve, the PPV of high Z-score and high fetal fraction is higher in groups of Z-score > the optimal cutoff value.
Conclusion
The results of this study show that the fetal fraction is significantly correlated with the PPV. Combining fetal fraction with Z-score is significantly better than in groups of Z-score-associated criteria; clinicians can give more accurate and efficient prenatal genetic counseling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Since 1997, the discovery of cell-free fetal DNA (cffDNA) has opened a new avenue for noninvasive prenatal testing (NIPT) [1]. Due to the wide application of the next-generation sequencing (NGS), NIPT has been widely used in the screening of fetal trisomies of 21, 18, and 13 [2,3,4,5]. Although NIPT has been proven to be currently the best noninvasive method for the diagnosis of common fetal aneuploidy, it remains a screening tool because cffDNA is mainly from the placenta rather than the fetus. There may be a discrepancy in genetic material between the placenta and the fetus [6, 7]. Therefore, further confirmation of invasive prenatal diagnosis is necessary.
Z-score represents the risk value of NIPT; Z-score (also called standard score) describes the position of a raw score in terms of its distance from the mean. The Z-score refers to the number of standard deviations from the mean of a reference data set [8]. A significant correlation between Z-score and positive predictive value (PPV) has been reported in many literatures [9], [10]. Some literatures also suggests that fetal fraction is crucial to the accuracy of the results [11, 12]. Indicating that accurate assessment of cffDNA concentration is an important prerequisite.
In this study, semiconductor sequencing platform with average read length of over 100 bp was used to sequence the complete cell-free DNA fragments in the peripheral blood [13]. In addition, fetal fraction can be calculated without being restricted by fetal gender [14]. To study the internal relationship between fetal fraction and PPV. Logistic regression and receiver operating characteristic (ROC) curve were adopted to evaluate the Z-score accuracy of NIPT for trisomies 21, 18, and 13 and to analyze the relationship between Z-score and PPV performance. The associations between Z-score and other parameters were analyzed to discover relevant factors.
Materials and methods
Participant recruitment
This study was approved by the Ethics Review Committee of Guangdong Women and Children Hospital (number 2013102301). All clinical work procedures are carried out in accordance with the relevant guidelines and practices. In consultation with physicians, subjects were informed of the objectivity, accuracy, consequence, and limitation of NIPT, and each participant signed an informed consent.
A total of 61,581 pregnant women who undergone NIPT in Guangdong Woman and Children Hospital from January 2015 to December 2020 were recruited for the retrospective study. After detailed genetic counseling by the doctor, according to their own conditions, pregnant women voluntarily choose 0.15 × NIPT or 0.4 × NIPT (NIPT-plus).
Sample preparation, DNA sequencing, and bioinformatics analysis
For each subject, a peripheral blood sample (5 ml) was withdrawn from the cubital vein using an EDTA K2 tube. The blood was centrifuged at 4 °C and 1,600 × g for 10 min by Eppendorf 5810R (Eppendorf, Germany). Then the plasma was transferred to another tube and centrifuged at 4 °C and 16,000 × g for 10 min by Eppendorf 5424 centrifuge (Eppendorf, Germany). The supernatant were stored at − 20 °C as soon as possible until genomic DNA is extracted.
DNA enrichment was performed after end-repairing with an average particle size of 1 um for the purpose of selecting fragments smaller than 160 bp [14]. The tubes were vibrated for 3 s after adding the beads. After 5 min, transfer the tubes to the magnetic rack. The supernatant was used for adaptor ligation.
The next-generation sequencing was performed using semiconductor sequencing technique on the Bioelectronseq 4000 sequencing platform (CFDA registration permit NO. 20153400309, CapitalBio, China). As detailed in our previous article [13], a total of 9–23 libraries were pooled and sequenced in the range of ~ 200 bp reads. This study included two different sequencing depth methods. The sequencing depths of NIPT and are about 0.15 × and 0.4 × , and the data volumes are 3 million and 8 million of reads, respectively.
Combined GC correction and Z-score testing methods were used to identify fetal chromosome aneuploidy of trisomies 21, 18, and 13 as described previously [13]. The fetal and maternal chromosome aneuploidy was classified using modified Stouffer’s Z-score method. The Z‐score obeyed standard normal distribution; Z-scores were used to evaluate the risk of chromosomal aneuploidies which was set within the range from − 3 to 3. If Z‐score > 3 or Z‐score < − 3, the sample was classified as a high risk of chromosomal aneuploidies. On the contrary, if the Z‐score was between − 3 and 3, the sample was divided to have a low risk.
To estimate the ratio of cffDNA in maternal peripheral blood, two types of methods were used [14]. Briefly, read proportion of the Y chromosome was used for a male fetus, and the LOESS regression was applied for a female fetus.
Prenatal diagnosis and pregnancy follow-up
Professional genetic counseling was given for pregnant women of high risk in NIPT. After fully informed, prenatal diagnosis to obtain fetal cells through puncture (villus, amniotic fluid, or umbilical cord blood) for fetal chromosome analysis was conducted. Patients at high risk for NIPT were confirmed by karyotyping (G-band resolution 400 bands) or chromosome microarray analysis (CMA) (CytoScanTM 750 K, Affymetrix, USA). All participants were followed up through telephone interviews for neonatal outcomes and growths.
Statistics
SPPS 22.0 were used for data statistical analysis. Logistic regression was used to analyze the relationship between the Z-score and the PPV performance. Python language was applied to carry on logistic regression analyses. The differences between rates were tested by Chi‐square test or Fisher exact tests. Results with P values < 0.05 were considered statistically significant. The associated criteria, specificity, and sensitivity were calculated by ROC curve analyses using MedCalc (Wan et al., 2021; Zhou et al., 2021). Correlation between Z-score and maternal age, gestational age, fetal fraction, or BMI was conducted by rank analyses using SPSS.
Results
Population profiles and NIPT results
Among the 61,581 cases who underwent NIPT, 49,393 adopted 0.15 × sequencing (NIPT), and 12,188 adopted 0.4 × sequencing (NIPT-plus). The basic characteristics and general results were listed in Table 1. A total of 377 were found to be trisomy positive and underwent invasive prenatal diagnosis of chromosomal microarray analysis (CMA) or chromosome karyotyping (CS). Among them, 278 were confirmed to have fetuses with trisomy, in which 213 were T21, 48 were T18, and 17 were T13. Under set conditions, the overall PPV was 73.74%. The PPV of T21, T18, and T13 were 84.80%, 69.23%, and 25.00% for NIPT, and the PPV of T21, T18, and T13 were 86.96%, 80.00%, and 35.00% for NIPT-plus.
Analysis of logistics regression and ROC curve
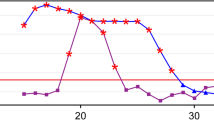
As shown in Fig. 1, the fitted logistic regression models revealed that Z-scores of NIPT/NIPT-plus positive results were positively related to true positive results (P < 0.05). For NIPT, ROC curve analysis showed that the optimal cutoff value of Z-scores for predication of T21, T18, and T13 was 7.597, 4.944, and 9.135; the areas under the ROC curves were 0.891, 0.928, and 0.796; and the Youden index J was 0.6794, 0.9141, and 0.6157, respectively (Fig. 2 and Table 2). The results showed that the sensitivity and specificity for T21 were 87.94% and 80%, for T18 were 96.94% and 94.44%, and for T13 were 87.50% and 74.07%. For NIPT-plus, ROC curve analysis showed that the optimal truncation value of Z-scores for predication of T21, T18, and T13 was 9.489, 8.004, and 12.4; the areas under the ROC curves were 0.762, 0.955, and 0.905; and the Youden index J was 0.5000, 0.9091, and 0.7738, respectively (Fig. 2 and Table 2). The results showed that the sensitivity and specificity of T21 were 92.86% and 57.14%, the sensitivity and specificity of T18 were 90.91% and 100%, and the sensitivity and specificity of T13 were 85.71% and 91.67%.
The ROC curve analysis of T21, T18, and T13. For NIPT, the areas under the ROC curves were 0.891, 0.928, and 0.796, The results showed that the sensitivity and specificity for T21 were 87.94% and 80%, for T18 were 96.94% and 94.44%, and for T13 were 87.50% and 74.07%. For NIPT-plus, the areas under the ROC curves were 0.762, 0.955, and 0.905, The results showed that the sensitivity and specificity of T21 were 92.86% and 57.14%, the sensitivity and specificity of T18 were 90.91% and 100%, and the sensitivity and specificity of T13 were 85.71% and 91.67%. NIPT, noninvasive prenatal testing.
Correlation analysis between Z-score and relative parameters
As Z-score was significantly correlated with PPV, the correlation between Z-score and other parameters were further studied. Rank correlation analysis between Z-score and maternal age, gestational age, fetal fraction, or BMI was conducted (Table 3). The spearman’s coefficient of rank correlation of between Z-score and fetal fraction in T21 of NIPT and NIPT-plus was 0.60 (P < 0.0001, 95% CI 0.49–0.69) and 0.82 (P < 0.0001, 95% CI 0.70–0.90). The Spearman’s coefficient of rank correlation between Z-score and BMI in T21 of NIPT was − 0.28 (P = 0.0001, 95% CI − 0.414– − 0.141). These results indicate that the fetal fraction was significantly correlated with Z-score in T21 of NIPT and NIPT-plus. A weak correlation was found between Z-score and BMI in T21 of NIPT. No significant correlation was found in T18 and T13 of NIPT and NIPT-plus, which may be due to the small sample size. It is worth mentioning that no significant correlation was found between fetal fraction and PPV.
Based on the above results, fetal fraction was taken into consideration when calculating the PPV based on Z-score. Due to the few data of T18 and T13, only T21 of NIPT and NIPT-plus were analyzed. The cases were first divided into two groups based on the associated criterion of Z-scores: the low Z-score (LZ) (Z-score < associated criterion) and high Z-score (HZ) groups (Z-score ≥ associated criterion). Then, the cases were divided into two groups based on associated criterion of ROC curves: the low criterion (LC (fetal fraction < associated criterion) and high criterion (HC) groups (fetal fraction ≥ associated criterion). The PPV of each group was calculated (Table 4). Nonparametric test of Mann–Whitney was used to test the difference significance between LC and HC, and Z and P values were shown. Compared with LC groups, HC groups have a higher PPV in HZ groups of NIPT and NIPT-plus. But no significant difference was found between LC groups and HC groups. No similar results were found between the HC and LC groups in LZ groups of NIPT and NIPT-plus.
Discussion
NIPT has been widely used in prenatal screening with excellent specificity and sensitivity [15]. PPV is often used to evaluate the accuracy of NIPT performance [16]. However, detailed genetic counseling before and after testing is much more important. Pre-test consultation can be carried out according to relevant international and domestic guidelines [17]. But it is difficult for doctors to give appropriate judgment for every high-risk outcome in post-test consultation.
In this study, both NIPT and NIPT-plus showed excellent sensitivity and specificity. Logistic regression and ROC analysis showed that the PPV of NIPT and NIPT-plus was significantly correlated with Z-score, which is consistent with other reports [9, 10]. Therefore, clinicians can first give effective and objective consultation opinions according to Z-score for high-risk patients.
Further, by analyzing the correlation between Z-score and other parameters, fetal fraction was found to be significantly correlated with the PPV in T21. Dividing the test results into two groups according to cutoff values of fetal fraction and Z-score showed a clear distinction of positive predictive value. No significant difference was found, which might due to insufficient statistical power. The fetal fraction is directly related to PPV, suggesting that higher fetal fraction enables to obtain more valid data and further more accurate results. Therefore, fetal fraction can be used as another reference following Z-Score for doctors’ post-test consultation. In addition, despite numerous reported that the fetal fraction increases with gestational age [18], no correlation between gestational age and Z-score was found in this study.
As cffDNA in maternal plasma was shorter than maternal cfDNA, some literatures reported that sequence shorter cfDNA fragments decreases the false negative rate of noninvasive prenatal testing [19, 20]. Therefore, the proportion of cell‐free fetal DNA (cffDNA) in extracted DNA is very important for NIPT result. In this study, cffDNA enrichment technology was used to increase the average cffDNA concentration from 10 to 20% and significantly reduce false negatives [14]. When the Z-score is between 3 and 5, the PPV of T21 high-risk is less than 20%. In theory, the chance of overturning is greater than 80%. In addition, it is also very important to evaluate the risk by combing with the fetal fraction. For example, the fetal fraction is 30%, but the Z value is only more than 4 points. The possibility of placental mosaicism is more higher [21], and the probability that the fetus itself is normal is relatively high. False positives and false negatives are unavoidable problems for NIPT [22]. Fetal cell-free DNA comes from the placenta, which develops from embryonic trophoblast cells. Due to the different cell sources of the placenta and fetal DNA, there is inconsistency in DNA materials. This phenomenon is called confined placental mosaicism (CPM), and the incidence of CPM can reach 1–3% [23].
Although many studies reported that the PPV of NIPT was significantly correlated with Z-score, few studies have involved fetal fraction. This study proposes that the fetal fraction is significantly correlated with the PPV, especially trisomy 21. The effect of fetal fraction was more pronounced in the low Z-score group. Combined with fetal fraction and Z-score analysis, clinicians can give more pertinent recommendations. The shortcoming of this study is that the sample size of the low Z-score group is small. The next plan is to accumulate more data for analysis and further confirm the current results.
In a word, there is a significant correlation between fetal fraction and PPV of NIPT. Combining fetal fraction with Z-score is significantly better than in groups of Z-score-associated criteria; clinicians can give more accurate and efficient prenatal genetic counseling.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- NIPT :
-
Noninvasive prenatal testing
- PPV :
-
Positive predictive value
- cffDNA :
-
Cell-free fetal DNA
- ACMG :
-
American Society of Medical Genetics and Genomics
- CMA :
-
Chromosome microarray analysis
- CS :
-
Chromosome karyotyping
- NGS :
-
Next-generation sequencing
- SSP :
-
Semiconductor sequencing platform
References
Lo YM, Corbetta N, Chamberlain PF, Rai V, Sargent IL, Redman CW, et al. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997;350:485–7. https://doi.org/10.1016/s0140-6736(97)02174-0.
Hu H, Wang L, Wu J, Zhou P, Fu J, Sun J, et al. (2019) Noninvasive prenatal testing for chromosome aneuploidies and subchromosomal microdeletions/microduplications in a cohort of 8141 single pregnancies. Hum Genomics 13(1). https://doi.org/10.1186/s40246-019-0198-2.
Kaseniit KE, Hogan GJ, D'Auria KM, Haverty C, Mu Zzey D (2018) Strategies to minimize false positives and interpret novel microdeletions based on maternal copy-number variants in 87,000 noninvasive prenatal screens. BMC Med Genomics 11. https://doi.org/10.1186/s12920-018-0410-6.
Liu Y, Liu H, He Y, Xu W, Ma Q, He Y, Yu F. Clinical performance of non-invasive prenatal served as a first-tier screening test for trisomy 21, 18, 13 and sex chromosome aneuploidy in a pilot city in China. Hum Genomics. 2020;14:1–9. https://doi.org/10.1186/s40246-020-00268-2.
Liao C, Yin AH, Peng CF, Fu F, Yang JX, Li R, ... Zhang K. Noninvasive prenatal diagnosis of common aneuploidies by semiconductor sequencing. Proc Natl Acad Sci. 2014;111(20):7415-7420.https://doi.org/10.1073/pnas.1321997111/-/DCSupplemental.
Neofytou M. Predicting fetoplacental mosaicism during cfDNA-based NIPT. Curr Opin Obstet Gynecol. 2020;32(2):152–8. https://doi.org/10.1097/GCO.0000000000000610.
Qi Y, Yang J, Hou Y, Guo F, Peng H, Wang D, ... Yin A (2019) The significance of trisomy 7 mosaicism in noninvasive prenatal screening. Hum Genomics 13(1):1-10.https://doi.org/10.1186/s40246-019-0201-y.
Chiu RWK, Chan KCA, Gao Y, Lau VYM, Zheng W, Leung TY, .... Lo YMD. Noninvasive prenatal diagnosis of fetal chromosomal aneuploidy by massively parallel genomic sequencing of DNA in maternal plasma. Proc Natl Acad Sci U S A. 2008;23;105(51):20458–63. https://doi.org/10.1073/pnas.0810641105.
Wan J, Li R, Yu Q, Wang Dan, Sun X, Zhang Y, Liao C. Evaluation of the Z-score accuracy of noninvasive prenatal testing for fetal trisomies 13, 18 and 21 at a single center. Prenat Diagn. 2021;41(6):690–6. https://doi.org/10.1002/pd.5908.
Zhou L, Zhang B, Liu J, Shi Y, Yu B. The optimal cutoff value of z-scores enhances the judgment accuracy of noninvasive prenatal screening. Front Genet. 2021;12:690063. https://doi.org/10.3389/fgene.2021.690063.
Xue Y, Zhao G, Qiao L, Lu J, Yu B, Wang T. Sequencing shorter cfDNA fragments decreases the false negative rate of non-invasive prenatal testing. Front Genet. 2020;11:280. https://doi.org/10.3389/fgene.2020.00280.
Qiao L, Yu B, Liang Y, Zhang C, Wu X, Xue Y, Wang T. Sequencing shorter cfDNA fragments improves the fetal DNA fraction in noninvasive prenatal testing. Am J Obstet Gynecol. 2019;221(4):345-e1. https://doi.org/10.1016/j.ajog.2019.05.023.
Hu H, Liu H, Peng C, Deng T, Fu X, Chung C, Yang Y. Clinical experience of non-invasive prenatal chromosomal aneuploidy testing in 190,277 patient samples. Curr Mol Med. 2016;16(8):759–66. https://doi.org/10.2174/1566524016666161013142335.
Hu P, Liang D, Chen Y, Lin Y, Qiao F, Li H, ... Xu Z. An enrichment method to increase cell-free fetal DNA fraction and significantly reduce false negatives and test failures for non-invasive prenatal screening: a feasibility study. J Transl Med. 2019;17(1):1-9.https://doi.org/10.1186/s12967-019-1871-x.
Yang L, Tan WC. Prenatal screening in the era of non-invasive prenatal testing: a Nationwide cross-sectional survey of obstetrician knowledge, attitudes and clinical practice. BMC Pregnancy Childbirth. 2020;20(1):1–8. https://doi.org/10.1186/s12884-020-03279-y.
Meck JM, Dugan EK, Matyakhina L, Aviram A, Trunca C, Pineda-Alvarez D, Cherry AM. Noninvasive prenatal screening for aneuploidy: positive predictive values based on cytogenetic findings. Am J Obstet Gynecol. 2015;213(2):214-e1. https://doi.org/10.1016/j.ajog.2015.04.001.
Rose NC, Kaimal AJ, Dugoff L, Norton ME, American College of Obstetricians and Gynecologists. Screening for fetal chromosomal abnormalities: ACOG practice bulletin, number 226. Obstet Gynecol. 2020;136(4):e48–69. https://doi.org/10.1097/AOG.0000000000004084.
Smith JF, Blumenfeld Y. Cell-free fetal DNA in maternal plasma: progress and potential. NeoReviews. 2008;9(8):e332–7. https://doi.org/10.1542/neo.9-8-e332.
Qiao L, Yu B, Liang Y, Zhang C, Wu X, Xue Y, ... Wang T. Sequencing shorter cfDNA fragments improves the fetal DNA fraction in noninvasive prenatal testing. Am J Obstet Gynecol. 2019;221(4):345-e1.https://doi.org/10.1016/j.ajog.2019.05.023.
Xue Y, Zhao G, Qiao L, Lu J, Yu B, Wang T. Sequencing shorter cfDNA fragments decreases the false negative rate of non-invasive prenatal testing. Front Genet. 2020;11:280. https://doi.org/10.3389/fgene.2020.00280.
Brison N, Neofytou M, et al. Predicting fetoplacental chromosomal mosaicism during non-invasive prenatal testing. Prenat Diagn. 2018;38(4):258–66. https://doi.org/10.1002/pd.5223.
Suzumori N, Sekizawa A, Sago H, et al. Retrospective details of false-positive and false-negative results in non-invasive prenatal testing for fetal trisomies 21, 18 and 13. Eur J Obstet Gynecol Reprod Biol. 2021;256:75–81. https://doi.org/10.1016/j.ejogrb.2020.10.050.
Grati FR, Malvestiti F, Ferreira JC, et al. Fetoplacental mosaicism: potential implications for false-positive and false-negative noninvasive prenatal screening results[J]. Genet Med. 2014;16:620–4. https://doi.org/10.1038/gim.2014.3.
Funding
This work was supported by Guangdong Province Basic and Applied Basic Research Funding Project (grant no. 2020A1515110779) and Guangdong Province Basic and Applied Basic Research Funding Project (2019A1515111000) and Guangzhou Basic and Applied Basic Research Funding Project (grant no. 202102080015).
Author information
Authors and Affiliations
Contributions
All authors have materially participated in the study and manuscript preparation. Jiexia Yang collected all clinical data and drafted the manuscript; Jing Wu and Dongmei Wang carried out all the molecular genetic analyses; Qi Zhang, Yaping Hou, Fangfang Guo, Haishan Peng, and Yixia Wang participated in the data analysis; and Aihua Yin designed the work and revised the manuscript. All authors have approved the final article.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Ethics Committee of Guangdong Women and Children Hospital.
Consent for publication
The authors declare that they have no competing interests, and the patients in this case report provided their consent for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yang, J., Wu, J., Wang, D. et al. Combined fetal fraction to analyze the Z-score accuracy of noninvasive prenatal testing for fetal trisomies 13, 18, and 21. J Assist Reprod Genet 40, 803–810 (2023). https://doi.org/10.1007/s10815-022-02694-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-022-02694-8