Abstract
Purpose
To enhance the in vitro growth of porcine oocytes, we studied the effect of mural granulosa cells (MGCs) on the viability of oocytes attached to granulosa cells (oocyte-granulosa cell complexes, OGCs) that were obtained from early antral follicles.
Methods and results
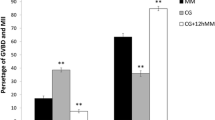
When OGCs were cultured with MGCs for 12 days, there were significant improvement (P < 0.05) in the robustness of gap junctional communication between the oocyte and the granulosa cells (82% vs. 59%), the survival rate of oocytes (57% vs. 39%), and the diameter of survived oocytes (118 μm vs. 112 μm). The rate of oocyte release of OGCs cultured with MGCs on the 12th day (1.9%) was significantly lower than that of OGCs cultured without MGCs (26%). Complete meiotic arrest was maintained in the group with MGCs (100%), while partial resumption of spontaneous meiosis was noticed in the absence of MGCs (10–19%). Furthermore, the presence of MGCs increased the oocyte maturation rate after maturation culture in both 12- and 14-day culture groups (P < 0.05, 85–88%) compared to OGCs cultured without MGCs (48–60%). MGCs also significantly improved the blastocyst formation rate (day 7) after ICSI (P < 0.05).
Conclusions
The data of this study thus shows that the presence of MGCs during in vitro oocyte growth plays a crucial role in supporting the developmental competence of growing porcine oocytes attached to the granulosa cells via enhancement of their viability.





Similar content being viewed by others
Data and materials availability
The data supporting the findings of the current study are available from the corresponding author upon request.
References
Mayo K, Jameson L, Woodruff TK. Eggs in the nest. Endocrinology. 2007;148:3577–9.
Mhawi AJ, Kaňka J, Motlík J. Follicle and oocyte growth in early postnatal calves: cytochemical, autoradiographical and electron microscopical studies. Reprod Nutr Dev. 1991;31:115–26.
Wigglesworth K, Lee KB, Emori C, Sugiura K, Eppig JJ. Transcriptomic diversification of developing cumulus and mural granulosa cells in mouse ovarian follicles. Biol Reprod. 2015;92:1–14.
Kidder GM, Vanderhyden BC. Bidirectional communication between oocytes and follicle cells: ensuring oocyte developmental competence. Can J Physiol Pharmacol. 2010;88:399–413.
Jaffe LA, Egbert JR. Regulation of mammalian oocyte meiosis by intercellular communication within the ovarian follicle. Annu Rev Physiol. 2017;79:237–60.
Anderson E, Albertini DF. Gap junctions between the oocyte and companion follicle cells in the mammalian ovary. J Cell Biol. 1976;71:680–6.
Racowsky C, Satterlie RA. Metabolic, fluorescent dye and electrical coupling between hamster oocytes and cumulus cells during meiotic maturation in vivo and in vitro. Dev Biol. 1985;108:191–202.
Eppig JJ. Intercommunication between mammalian oocytes and companion somatic cells. BioEssays. 1991;13:569–74.
Zhang M, Su YQ, Sugiura K, Xia G, Eppig JJ. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science. 2010;330:366–9.
Tuji T, Kiyosu C, Akiyama K, Kunieda T. CNP/NPR2 signaling maintains oocyte meiotic arrest in early antral follicles and is suppressed by EGFR-mediated signaling in preovulatory follicles. Mol Reprod Dev. 2012;79:795–802.
Hirao Y, Nagai T, Kubo M, Miyano T, Miyake M, Kato S. In vitro growth and maturation of pig oocytes. J Reprod Fertil. 1994;100:333–9.
Telfer EE. The development of methods for isolation and culture of preantral follicles from bovine and porcine ovaries. Theriogenology. 1996;45:101–10.
Wu J, Emery BR, Carrell DT. In vitro growth, maturation, fertilization, and embryonic development of oocytes from porcine preantral follicles. Biol Reprod. 2001;64:375–81.
Mao J, Wu G, Smith MF, McCauley TC, Cantley TC, Prather RS, et al. Effects of culture medium, serum type, and various concentrations of follicle-stimulating hormone on porcine preantral follicular development and antrum formation in vitro. Biol Reprod. 2002;67:1197–203.
Hashimoto S, Ohsumi K, Tsuji Y, Harauma N, Miyata Y, Fukuda A, et al. Growing porcine oocyte-granulosa cell complexes acquired meiotic competence during in vitro culture. J Reprod Dev. 2007;53:379–84.
Wu J, Xu B, Wang W. Effects of luteinizing hormone and follicle stimulating hormone on the developmental competence of porcine preantral follicle oocytes grown in vitro. J Assist Reprod Genet. 2007;24:419–24.
Tasaki H, Iwata H, Sato D, Monji Y, Kuwayama T. Estradiol has a major role in antrum formation of porcine preantral follicles cultured in vitro. Theriogenology. 2013;79:809–14.
Kubo N, Cayo-Colca IS, Miyano T. Effect of estradiol-17β during in vitro growth culture on the growth, maturation, cumulus expansion and development of porcine oocytes from early antral follicles. Anim Sci J. 2015;86:251–9.
Itami N, Munakata Y, Shirasuna K, Kuwayama T, Iwata H. Promotion of glucose utilization by insulin enhances granulosa cell proliferation and developmental competence of porcine oocyte grown in vitro. Zygote. 2017;25:65–74.
Munakata Y, Sugimoto A, Shirasuna K, Kuwayama T, Iwata H. Xanthan gum and Locust bean gum gel supports in vitro development of porcine oocytes derived from early antral follicles. J Reprod Dev. 2019;65:551–4.
Cayo-Colca IS, Yamagami Y, Phan TC, Miyano T. A combination of FSH and dibutyryl cyclic AMP promote growth and acquisition of meiotic competence of oocytes from early porcine antral follicles. Theriogenology. 2011;75:1602–12.
Sugimoto H, Kida Y, Miyamoto Y, Kitada K, Matsumoto K, Saeki K, et al. Growth and development of rabbit oocytes in vitro: effect of fetal bovine serum concentration on culture medium. Theriogenology. 2012;78:1040–7.
Hirao Y, Itoh T, Shimizu M, Iga K, Aoyagi K, Kobayashi M, et al. In vitro growth and development of bovine oocyte-granulosa cell complexes on the flat substratum: effects of high polyvinylpyrrolidone concentration in culture medium. Biol Reprod. 2004;70:83–91.
Eppig JJ, Wigglesworth K. Factors affecting the developmental competence of mouse oocytes grown in vitro: oxygen concentration. Mol Reprod Dev. 1995;42:447–56.
Hirao Y, Shimizu M, Iga K, Takenouchi N. Optimization of oxygen concentration for growing bovine oocytes in vitro: constant low and high oxygen concentrations compromise the yield of fully grown oocytes. J Reprod Dev. 2012;58:204–11.
Hirao Y, Shimizu M, Iga K, Takenouchi N. Growth of bovine oocyte-granulosa cell complexes cultured individually in microdrops of various sizes. J Reprod Dev. 2009;55:88–93.
Yamochi T, Hashimoto S, Yamanaka M, Nakaoka Y, Morimoto Y. Optimum culture duration for growing oocytes to attain meiotic and fertilization competence. J Reprod Dev. 2017;63:591–5.
Wang WH, Abeydeera LR, Okuda K, Niwa K. Penetration of porcine oocytes during maturation in vitro by cryopreserved, ejaculated spermatozoa. Biol Reprod. 1994;50:510–5.
Abeydeera LR, Day BN. In vitro penetration of pig oocytes in a modified Tris-buffered medium: effect of BSA, caffeine and calcium. Theriogenology. 1997;48(4):537–44.
Hashimoto S. Application of in vitro maturation to assisted reproductive technology. J Reprod Dev. 2009;55:1–10.
Dieci C, Lodde V, Franciosi F, Lagutina I, Tessaro I, Modina SC, et al. The effect of cilostamide on gap junction communication dynamics, chromatin remodeling, and competence acquisition in pig oocytes following parthenogenetic activation and nuclear transfer. Biol Reprod. 2013;89:68.
Yoshioka K, Suzuki C, Onishi A. Defined system for in vitro production of porcine embryos using a single basic medium. J Reprod Dev. 2008;54:208–13.
Pangas SA, Matzuk MM. Genetic models for transforming growth factor beta superfamily signaling in ovarian follicle development. Mol Cell Endocrinol. 2004;225:83–91.
McNatty KP, Juengel JL, Reader KL, Lun S, Myllymaa S, Lawrence SB, et al. Bone morphogenetic protein 15 and growth differentiation factor 9 co-operate to regulate granulosa cell function in ruminants. Reproduction. 2005;129:481–7.
Mottershead DG, Ritter LJ, Gilchrist RB. Signalling pathways mediating specific synergistic interactions between GDF9 and BMP15. Mol Hum Reprod. 2012;18:121–8.
Wang XL, Wang K, Zhao S, Wu Y, Gao H, Zeng SM. Oocyte-Secreted Growth Differentiation Factor 9 Inhibits BCL-2-Interacting Mediator of Cell Death-Extra Long Expression in Porcine Cumulus Cell. Biol Reprod. 2013;89:1–9.
Zhai B, Liu H, Li X, Dai L, Gao Y, Li C, et al. BMP15 prevents cumulus cell apoptosis through CCL2 and FBN1 in porcine ovaries. Cell Physiol Biochem. 2013;32:264–78.
Miyoshi T, Otsuka F, Nakamura E, Inagaki K, Ogura-Ochi K, Tsukamoto N, et al. Regulatory role of kit ligand-c-kit interaction and oocyte factors in steroidogenesis by rat granulosa cells. Mol Cell Endocrinol. 2012;358:18–26.
Hutt KJ, Mclaughlin EA, Holland MK. Kit ligand and c-Kit have diverse roles during mammalian oogenesis and folliculogenesis. Mol Hum Reprod. 2006;12:61–9.
Norris RP, Ratzan WJ, Freudzon M, Mehlmann LM, Krall J, Movsesian MA, et al. Cyclic GMP from the surrounding somatic cells regulates cyclic AMP and meiosis in the mouse oocyte. Development. 2009;136:1869–78.
Carabatsos MJ, Sellitto C, Goodenough DA, Albertini DF. Oocyte-granulosa cell heterologous gap junctions are required for the coordination of nuclear and cytoplasmic meiotic competence. Dev Biol. 2000;226:167–79.
Biggers JD, Whittingham DG, Donahue RP. The pattern of energy metabolism in the mouse oocyte and zygote. Proc Natl Acad Sci U S A. 1967;58:560–7.
Downs SM, Utecht AM. Metabolism of radiolabeled glucose by mouse oocytes and oocyte cumulus cell complexes. Biol Reprod. 1999;60:1446–52.
Eppig JJ, Pendola FL, Wigglesworth K, Pendola JK. Mouse oocytes regulate metabolic cooperativity between granulosa cells and oocytes: amino acid transport. Biol Reprod. 2005;73:351–7.
Fair T, Hyttel P, Greve T. Bovine oocyte diameter in relation to maturational competence and transcriptional activity. Mol Reprod Dev. 1995;42:437–42.
Anguita B, Jimenez-Macedo AR, Izquierdo D, Mogas T, Paramio MT. Effect of oocyte diameter on meiotic competence, embryo development, p34 (cdc2) expression and MPF activity in prepubertal goat oocytes. Theriogenology. 2007;67:526–36.
Hashimoto S, Saeki K, Nagao Y, Minami N, Yamada M, Utsumi K. Effects of cumulus cell density during in vitro maturation of the developmental competence of bovine oocytes. Theriogenology. 1998;49:1451–63.
Han ZB, Lan GC, Wu YG, Han D, Feng WG, Wang JZ, et al. Interactive effects of granulosa cell apoptosis, follicle size, cumulus–oocyte complex morphology, and cumulus expansion on the developmental competence of goat oocytes: a study using the well-in-drop culture system. Reproduction. 2006;132:749–58.
Munakata Y, Ueda M, Kawahara-Miki R, Kansaku K, Itami N, Shirasuna K, et al. Follicular factors determining granulosa cell number and developmental competence of porcine oocytes. J Assist Reprod Genet. 2018;35:1809–19.
Hirao Y. Isolation of ovarian components essential for growth and development of mammalian oocytes in vitro. J Reprod Dev. 2012;58:167–74.
Eppig JJ, Schroeder AC. Capacity of mouse oocytes from preantral follicles to undergo embryogenesis and development to live young after growth, maturation, and fertilization in vitro. Biol Reprod. 1989;41:268–76.
Munakata Y, Kawahara-Miki R, Shiratsuki S, Tasaki H, Itami N, Shirasuna K, et al. Gene expression patterns in granulosa cells and oocytes at various stages of follicle development as well as in in vitro grown oocyte-and-granulosa cell complexes. J Reprod Dev. 2016;62:359–66.
Acknowledgments
The authors thank Drs. USK Gamage and A. Takeshita for their helpful comments and M Brahmajosyula, PhD, for editing a draft of this manuscript.
Funding
Part of this work was supported by a grant from the Japan Society for the Promotion of Science (KAKENHI 20K09674 to S.H.) and IVF Japan group.
Author information
Authors and Affiliations
Contributions
TY and SH designed the experiment, interpreted the results, and wrote the manuscript with help from all authors. YM supervised the project.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
Supplementary figure Representative images of culturing oocytes. a Intact oocyte on day 10. b Shrunken oocyte on day 6. c Fragmented oocyte on day 6. The region of cell membrane is clearly visible under an interference phase contrast microscope even after granulosa cell proliferation. Bar: 20 μm (PNG 1725 kb).
ESM 2
(DOCX 24 kb).
Rights and permissions
About this article
Cite this article
Yamochi, T., Hashimoto, S. & Morimoto, Y. Mural granulosa cells support to maintain the viability of growing porcine oocytes and its developmental competence after insemination. J Assist Reprod Genet 38, 2591–2599 (2021). https://doi.org/10.1007/s10815-021-02212-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-021-02212-2