Abstract
Background
Strong evidence has suggested an important role of telomeres in meiosis, fertilization, and embryo development.
Purpose
To determine if sperm telomere length (STL) in sperm purified by differential gradient centrifugation followed by swim-up (selected STL) is correlated with sperm quality and clinical outcomes.
Methods
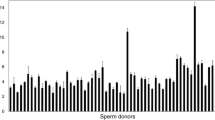
Relative selected STL was assessed by quantitative polymerase chain reaction (Q-PCR) in 78 consecutive assisted reproductive technology (ART) treatments during 2017. Statistical analyses were performed in the totality of patients, and in normozoospermic and non-normozoospermic patients. These included correlations between selected STL and sperm quality parameters, embryological parameters (multivariable linear regression), and clinical parameters (multivariable logistic regression).
Results
No significant correlations were found between selected STL and sperm quality in the total population. However, selected STL was significantly correlated with total sperm count (r = 0.361; P = 0.039) and sperm DNA fragmentation-post-acrosomal region pattern (r = − 0.464; P = 0.030) in normozoospermic patients. No relation was observed between selected STL and clinical outcomes in any clinical group.
Conclusions
As the correlations observed in normozoospermic patients were not representative of the whole heterogeneous population, differences in the sperm characteristics of the study population may lead to discrepant results when evaluating the association of STL with sperm quality. Since the total population selected STL was not related with sperm quality and with clinical outcomes, results do not support the use of selected STL measurement to evaluate the reproductive potential of the male patient or to predict the success rates of ART treatments.






Similar content being viewed by others
References
Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;1337.
Hamada A, Esteves SC, Agarwal A. Unexplained male infertility: potential causes and management. Hum Andrology. 2011;1(1):2–16.
Blackburn EH. Structure and function of telomeres. Nature. 1991;350(6319):569–73.
Scherthan H. Telomeres and meiosis in health and disease. Cell Mol Life Sci. 2007;64(2):117–24.
Klutstein M, Fennell A, Fernández-Álvarez A, Cooper JP. The telomere bouquet regulates meiotic centromere assembly. Nat Cell Biol. 2015;17(4):458–69.
Zalensky A, Zalenskaya I. Organization of chromosomes in spermatozoa: an additional layer of epigenetic information? Biochem Soc Trans. 2007;35(Pt 3):609–11.
Ioannou D, Millan NM, Jordan E, Tempest HG. A new model of sperm nuclear architecture following assessment of the organization of centromeres and telomeres in three-dimensions. Sci Rep. 2017;7:41585.
Samassekou O, Gadji M, Drouin R, Yan J. Sizing the ends: normal length of human telomeres. Ann Anat. 2010;192(5):284–91.
McEachern MJ, Krauskopf A, Blackburn EH. Telomeres and their control. Annu Rev Genet. 2000;34:331–58.
Forsyth NR, Wright WE, Shay JW. Telomerase and differentiation in multicellular organisms: turn it off, turn it on, and turn it off again. Differentiation. 2002;69(4–5):188–97.
de Lange T. How telomeres solve the end-protection problem. Science. 2009;326(5955):948–52.
Kozik A, Bradbury M, Zalensky A. Increased telomere size in sperm cells of mammals with long terminal (TTAGGG)n arrays. Mol Reprod Dev. 1998;51(1):98–104.
Brenner CA, Wolny YM, Adler RR, Cohen J. Alternative splicing of the telomerase catalytic subunit in human oocytes and embryos. Mol Hum Reprod. 1999;5(9):845–50.
Turner S, Hartshorne GM. Telomere lengths in human pronuclei, oocytes and spermatozoa. Mol Hum Reprod. 2013;19(8):510–8.
Schaetzlein S, Lucas-Hahn A, Lemme E, Kues WA, Dorsch M, Manns MP, et al. Telomere length is reset during early mammalian embryogenesis. Proc Natl Acad Sci U S A. 2004;101(21):8034–8.
Turner S, Wong HP, Rai J, Hartshorne GM. Telomere lengths in human oocytes, cleavage stage embryos and blastocysts. Mol Hum Reprod. 2010;16(9):685–94.
Varela E, Schneider RP, Ortega S, Blasco MA. Different telomere-length dynamics at the inner cell mass versus established embryonic stem (ES) cells. Proc Natl Acad Sci U S A. 2011;108(37):15207–12.
Baird DM, Britt-Compton B, Rowson J, Amso NN, Gregory L, Kipling D. Telomere instability in the male germline. Hum Mol Genet. 2006;15(1):45–51.
Lopes AC, Oliveira PF, Sousa M. Shedding light into the relevance of telomeres in human reproduction and male factor infertility. Biol Reprod. 2019;100(2):318–30.
Cariati F, Jaroudi S, Alfarawati S, Raberi A, Alviggi C, Pivonello R, et al. Investigation of sperm telomere length as a potential marker of paternal genome integrity and semen quality. Reprod BioMed Online. 2016;33(3):404–11.
Rocca MS, Speltra E, Menegazzo M, Garolla A, Foresta C, Ferlin A. Sperm telomere length as a parameter of sperm quality in normozoospermic men. Hum Reprod. 2016;31(6):1158–63.
Zhao F, Yang Q, Shi S, Luo X, Sun Y. Semen preparation methods and sperm telomere length: density gradient centrifugation versus the swim up procedure. Sci Rep. 2016;639051.
Lafuente R, Bosch-Rue E, Ribas-Maynou J, Alvarez J, Brassesco C, Amengual MJ, et al. Sperm telomere length in motile sperm selection techniques: a qFISH approach. Andrologia. 2018;50(2).
Thilagavathi J, Kumar M, Mishra SS, Venkatesh S, Kumar R, Dada R. Analysis of sperm telomere length in men with idiopathic infertility. Arch Gynecol Obstet. 2013;287(4):803–7.
Torra-Massana M, Barragan M, Bellu E, Oliva R, Rodriguez A, Vassena R. Sperm telomere length in donor samples is not related to ICSI outcome. J Assist Reprod Genet. 2018;35(4):649–57.
Bucar S, Goncalves A, Rocha E, Barros A, Sousa M, Sa R. DNA fragmentation in human sperm after magnetic-activated cell sorting. J Assist Reprod Genet. 2015;32(1):147–54.
Santiso R, Tamayo M, Gosalvez J, Meseguer M, Garrido N, Fernandez JL. Swim-up procedure selects spermatozoa with longer telomere length. Mutat Res. 2010;688(1–2):88–90.
Yang Q, Zhang N, Zhao F, Zhao W, Dai S, Liu J, et al. Processing of semen by density gradient centrifugation selects spermatozoa with longer telomeres for assisted reproduction techniques. Reprod BioMed Online. 2015;31(1):44–50.
Rooney DE, Czepulkowski BH. Human chromosome preparation: essential techniques. New York: Wiley; 1997.
World Health Organization. WHO laboratory manual for the examination and processing of human semen. 5th ed. Geneva: WHO Press; 2010.
Huirne JA, Homburg R, Lambalk CB. Are GnRH antagonists comparable to agonists for use in IVF? Hum Reprod. 2007;22(11):2805–13.
Pinto F, Oliveira C, Cardoso MF, Teixeira-da-Silva J, Silva J, Sousa M, et al. Impact of GnRH ovarian stimulation protocols on intracytoplasmic sperm injection outcomes. Reprod Biol Endocrinol. 2009;75.
Tesarik J, Sousa M, Testart J. Human oocyte activation after intracytoplasmic sperm injection. Hum Reprod. 1994;9(3):511–8.
Tesarik J, Sousa M. Key elements of a highly efficient intracytoplasmic sperm injection technique: Ca2+ fluxes and oocyte cytoplasmic dislocation. Fertil Steril. 1995;64(4):770–6.
Vandervorst M, Liebaers I, Sermon K, Staessen C, De Vos A, Van de Velde H, et al. Successful preimplantation genetic diagnosis is related to the number of available cumulus–oocyte complexes. Hum Reprod. 1998;13(11):3169–76.
Gardner DK, Lane M, Stevens J, Schlenker T, Schoolcraft WB. Blastocyst score affects implantation and pregnancy outcome: towards a single blastocyst transfer. Fertil Steril. 2000;73(6):1155–8.
Radesic B, Tremellen K. Oocyte maturation employing a GnRH agonist in combination with low-dose hcg luteal rescue minimizes the severity of ovarian hyperstimulation syndrome while maintaining excellent pregnancy rates. Hum Reprod. 2011;26(12):3437–42.
Sá R, Cunha M, Rocha E, Barros A, Sousa M. Sperm DNA fragmentation is related to sperm morphological staining patterns. Reprod BioMed Online. 2015;31(4):506–15.
Gomes M, Goncalves A, Rocha E, Sa R, Alves A, Silva J, et al. Effect of in vitro exposure to lead chloride on semen quality and sperm DNA fragmentation. Zygote. 2015;23(3):384–93.
Sergerie M, Laforest G, Bujan L, Bissonnette F, Bleau G. Sperm DNA fragmentation: threshold value in male fertility. Hum Reprod. 2005;20(12):3446–51.
Zidi-Jrah I, Hajlaoui A, Mougou-Zerelli S, Kammoun M, Meniaoui I, Sallem A, et al. Relationship between sperm aneuploidy, sperm DNA integrity, chromatin packaging, traditional semen parameters, and recurrent pregnancy loss. Fertil Steril. 2016;105(1):58–64.
Majzoub A, Agarwal A, Cho CL, Esteves SC. Sperm DNA fragmentation testing: a cross sectional survey on current practices of fertility specialists. Transl Androl Urol. 2017;6(Suppl 4):S710–9.
Hofmann N, Hilscher B. Use of aniline blue to assess chromatin condensation in morphologically normal spermatozoa in normal and infertile men. Hum Reprod. 1991;6(7):979–82.
Sellami A, Chakroun N, Ben Zarrouk S, Sellami H, Kebaili S, Rebai T, et al. Assessment of chromatin maturity in human spermatozoa: useful aniline blue assay for routine diagnosis of male infertility. Adv Urol. 2013;2013578631.
Vegetti W, Van Assche E, Frias A, Verheyen G, Bianchi M, Bonduelle M, et al. Correlation between semen parameters and sperm aneuploidy rates investigated by fluorescence in-situ hybridization in infertile men. Hum Reprod. 2000;15(2):351–65.
Turnpenny P, Ellard S. Emery’s elements of medical genetics. 14th ed. Philadelphia: Elsevier Churchil Livingstone; 2012.
Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47.
Covault J, Abreu C, Kranzler H, Oncken C. Quantitative real-time PCR for gene dosage determinations in microdeletion genotypes. Biotechniques. 2003;35(3):594–8.
O’Callaghan N, Dhillon V, Thomas P, Fenech M. A quantitative real-time PCR method for absolute telomere length. Biotechniques. 2008;44(6):807–9.
Bernardino RL, Dias TR, Moreira BP, Cunha M, Barros A, Oliveira E, et al. Carbonic anhydrases are involved in mitochondrial biogenesis and control the production of lactate by human sertoli cells. FEBS J. 2019;286(7):1393–406.
Dias TR, Alves MG, Bernardino RL, Martins AD, Moreira AC, Silva J, et al. Dose-dependent effects of caffeine in human sertoli cells metabolism and oxidative profile: relevance for male fertility. Toxicology. 2015:32812–20.
Mukaka M. Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med J. 2012;24(3):69–71.
Hammer Ø, Harper D, Ryan P. Past: paleontological statistics software package for education and data analysis. Palaeontol Electronica. 2001;4(1):1–9.
Bray I, Gunnell D, Davey SG. Advanced paternal age: how old is too old? J Epidemiol Community Health. 2006;60(10):851–3.
Kimura M, Cherkas LF, Kato BS, Demissie S, Hjelmborg JB, Brimacombe M, et al. Offspring’s leukocyte telomere length, paternal age, and telomere elongation in sperm. PLoS Genet. 2008;4(2):e37.
Reig-Viader R, Capilla L, Vila-Cejudo M, Garcia F, Anguita B, Garcia-Caldes M, et al. Telomere homeostasis is compromised in spermatocytes from patients with idiopathic infertility. Fertil Steril. 2014;102(3):728–38 e1.
Ricci G, Perticarari S, Fragonas E, Giolo E, Canova S, Pozzobon C, et al. Apoptosis in human sperm: its correlation with semen quality and the presence of leukocytes. Hum Reprod. 2002;17(10):2665–72.
Hemann MT, Rudolph KL, Strong MA, DePinho RA, Chin L, Greider CW. Telomere dysfunction triggers developmentally regulated germ cell apoptosis. Mol Biol Cell. 2001;12(7):2023–30.
Liu L, Franco S, Spyropoulos B, Moens PB, Blasco MA, Keefe DL. Irregular telomeres impair meiotic synapsis and recombination in mice. Proc Natl Acad Sci U S A. 2004;101(17):6496–501.
Yang Q, Zhao F, Dai S, Zhang N, Zhao W, Bai R, et al. Sperm telomere length is positively associated with the quality of early embryonic development. Hum Reprod. 2015;30(8):1876–81.
Kidd SA, Eskenazi B, Wyrobek AJ. Effects of male age on semen quality and fertility: a review of the literature. Fertil Steril. 2001;75(2):237–48.
Hassan MAM, Killick SR. Effect of male age on fertility: evidence for the decline in male fertility with increasing age. Fertil Steril. 2003:791520–7.
Aston KI, Hunt SC, Susser E, Kimura M, Factor-Litvak P, Carrell D, et al. Divergence of sperm and leukocyte age-dependent telomere dynamics: implications for male-driven evolution of telomere length in humans. Mol Hum Reprod. 2012;18(11):517–22.
Allsopp R, Vaziri H, Patterson C, Goldstein S, Younglai E, Futcher A, et al. Telomere length predicts replicative capacity of human fibroblasts. Proc Natl Acad Sci U S A. 1992;89(21):10114–8.
Singh NP, Muller CH, Berger RE. Effects of age on DNA double-strand breaks and apoptosis in human sperm. Fertil Steril. 2003;80(6):1420–30.
Schmid TE, Eskenazi B, Baumgartner A, Marchetti F, Young S, Weldon R, et al. The effects of male age on sperm DNA damage in healthy non-smokers. Hum Reprod. 2007;22(1):180–7.
Agarwal A, Ikemoto I, Loughlin KR. Relationship of sperm parameters with levels of reactive oxygen species in semen specimens. J Urol. 1994;152(1):107–10.
Pereira R, Sá R, Barros A, Sousa M. Major regulatory mechanisms involved in sperm motility. Asian J Androl. 2017;19(1):5–14.
Acknowledgments
We would like to acknowledge to the team of the IVF clinic: Jorge Beires, MD, and José Manuel Teixeira da Silva, MD, Gynecology, and Obstetrics (for oocyte retrieval); José Correia, MD, Anesthesiology (for anesthesia); Cristiano Oliveira, MD, José Teixeira da Silva, MD, Pedro Xavier, MD, António Couceiro MD, and Sandra Soares, MD, Gynecology and Obstetrics, subspecialty in Reproductive Medicine (for patient evaluation, controlled ovarian hyperstimulation, embryo transfer, and patient follow-up); Joaquina Silva, MD, Mariana Cunha, MSc, and Paulo Viana, MSc, Senior Clinical Embryologists (ESHRE), and Nuno Barros, MSc, Clinical Embryologist (for embryology laboratorial work); Ana Gonçalves, MSc, and Cláudia Osório, MSc (for andrology laboratorial work); Carolina Lemos, PhD (for additional statistical assistance).
Funding
UMIB (Pest-OE/SAU/UI0215/2014) is funded by the National Funds through FCT-Foundation for Science and Technology.
Author information
Authors and Affiliations
Contributions
A.C.L. was involved in performing molecular biology experimental procedures, acquisition of data, analysis and interpretation of data, and writing of the article. P.F.O. was involved in the supervision of molecular biology experimental procedures, analysis and interpretation of data, and critical approval of the final article. S.P. was involved in performing sperm preparation, semen analysis and embryological work, and critical approval of the final article. C.A. and M.J.P. were involved in performing the determination of sperm aneuploidies and critical approval of the final article. R.S. was involved in performing the determination of sperm chromatin maturity and sperm DNA fragmentation and critical approval of the final article. E.R was involved in the supervision of the statistical work and critical approval of the final article. A.B. was involved in patient recruitment and in the supervision of the IVF laboratory and critical approval of the final article. M.S. was involved in the conception and design of the study, analysis and interpretation of data, and final writing of the article.
Corresponding author
Ethics declarations
The procedures of the infertility clinic CGR A.Barros are under the determinations of the National Law on Medically Assisted Procreation (Law of 2017) and supervised by the National Council on Medically Assisted Procreation (CNPMA-2018). According to these rules and guidelines, the use of clinic databases and patient biological material for diagnosis and research can be used without further ethical approval, under strict individual anonymity, and after patient written informed consent. Regarding the use of semen samples for laboratorial experimentation at ICBAS-UP, the Ethics Committee authorization number is Project 2019/CE/P017 (266/CETI/ICBAS).
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
A draft of the present manuscript was awarded with the first Prize for the Best Basic Sciences Project by the Portuguese Society of Reproductive Medicine.
Electronic supplementary material
Supplementary Figure 1
Representative observations of the analysis of sperm samples. (a) TUNEL assay for sperm DNA fragmentation evaluation, considering the different staining patterns. Evaluation was performed in morphologically normal spermatozoa. Upper panel: (1) sperm head; (2) acrosome vesicle region; (3) post-acrosome region; (4) equatorial region. Lower panel: schematic representation of the same images of the upper panel, with the same corresponding letters, to facilitate interpretation. Scale bar = 10 μm. (b) Aniline blue (AB) assay for sperm chromatin maturity evaluation: (1): AB-negative spermatozoon, revealing sperm chromatin fully condensed; (2): AB-positive spermatozoon, revealing sperm immature chromatin condensation. Scale bar = 20 μm. (c) FISH assay for sperm chromosomic numeric alterations evaluation: (1) haploid (X,18); (2) haploid (Y,18); (3) sex chromosome nullisomy (18+); (4) chromosome 18 nullisomy (Y+); (5) chromosome 18 nullisomy (X+); (6) sex chromosome disomy (XX/18); (7) sex chromosome disomy (YY/18); (8) sex chromosome disomy (XY/18); (9) chromosome 18 disomy (X/18,18); (10) chromosome 18 disomy (Y/18,18); (11) sexual and autosomal disomy (XX/18,18); (12) sexual and autosomal disomy (XY/18,18). Color legends: X chromosome (green); Y chromosome (orange); chromosome 18 (aqua-blue); nucleus (DAPI-blue). Scale bar = 1 μm. (DOCX 931 kb)
Supplementary Figure 2
Frequency distribution histogram of relative STL in the total population (Total), and in cases with embryo transfer cycles (ETC), biochemical pregnancy (BP), clinical pregnancy (CP), ongoing pregnancy (OP) and live-birth delivery (LBD). (DOCX 19 kb)
Supplementary Figure 3
Comparison of relative sperm telomere length (STL) within clinical outcomes: negative, for no pregnancy; biochemical pregnancy only (BP); clinical pregnancy only (CP) and ongoing pregnancy (OP). Relative STL did not significantly differ among the different pregnancy status. a Total population: Kruskal-Wallis test, P = 0.257. Medians are represented. b Normozoospermic patients: Kruskal-Wallis test, P = 0.082. Medians are represented. c Non-normozoospermic patients: One-way ANOVA, P = 0.208. Means are represented. (DOCX 53 kb)
Supplementary Table 1
(DOCX 23 kb)
Supplementary Table 2
(DOCX 16 kb)
Supplementary Table 3
(DOCX 14 kb)
Rights and permissions
About this article
Cite this article
Lopes, A.C., Oliveira, P.F., Pinto, S. et al. Discordance between human sperm quality and telomere length following differential gradient separation/swim-up. J Assist Reprod Genet 37, 2581–2603 (2020). https://doi.org/10.1007/s10815-020-01897-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-01897-1