Abstract
Aim
To reveal whether there are differences in follicular fluid metabolomics profile of women with advanced maternal age (AMA).
Method
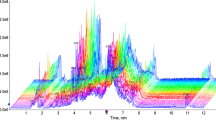
The group with advanced maternal age includes 23 patients above the age of 40, and the control group includes 31 patients aged between 25 and 35 years and AMH values above 1.1 ng/mL with no low ovarian response history. A single follicular fluid sample from a MII oocyte obtained during the oocyte pick-up procedure was analyzed with high-resolution 1H-NMR (nuclear magnetic resonance) spectroscopy. The results were evaluated using advanced bioinformatics analysis methods.
Results
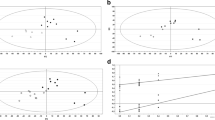
Statistical analysis of the NMR spectroscopy data from two groups showed that α-glucose and β-glucose levels of follicular fluid were decreased in the patients with AMA, while in contrast, lactate and trimethylamine N-oxide (TMAO) levels were increased in these patients compared with the controls. In addition to these, there was an increase in alanine levels and a decrease in acetoacetate levels in patients with AMA. However, these changes were not statistically significant.
Conclusion
Obtained results suggest that the follicular cell metabolism of patients with AMA is different from controls. These environmental changes could be associated with the low success rates of IVF treatment seen in these patients.




Similar content being viewed by others
References
Wood JW. Fecundity and natural fertility in humans. Oxf Rev Reprod Biol. 1989;11:61–109.
Te Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update. 2002;8(2):141–54.
Tatone C, Amicarelli F, Carbone MC, Monteleone P, Caserta D, Marci R, et al. Cellular and molecular aspects of ovarian follicle ageing. Hum Reprod Update. 2008;14(2):131–42.
Wright VC, Schieve LA, Reynolds MA, Jeng G. Assisted reproductive technology surveillance—United States, 2002. Morbidity and Mortality Weekly Report: Surveillance Summaries. 2005;54(2):1–24.
Katz-Jaffe MG, Surrey ES, Minjarez DA, Gustofson RL, Stevens JM, Schoolcraft WB. Association of abnormal ovarian reserve parameters with a higher incidence of aneuploid blastocysts. Obstet Gynecol. 2013;121(1):71–7.
Lekamge DN, Barry M, Kolo M, Lane M, Gilchrist RB, Tremellen KP. Anti-Müllerian hormone as a predictor of IVF outcome. Reprod BioMed Online. 2007;14(5):602–10.
Nikolaou D, Templeton A. Early ovarian ageing: a hypothesis: detection and clinical relevance. Hum Reprod. 2003;18(6):1137–9.
Sauer MV, Paulson RJ, Lobo RA. A preliminary report on oocyte donation extending reproductive potential to women over 40. N Engl J Med. 1990;323(17):1157–60.
Fortune JE. Ovarian follicular growth and development in mammals. Biol Reprod. 1994;50(2):225–32.
Nandi S, Kumar VG, Manjunatha BM, Gupta PSP. Biochemical composition of ovine follicular fluid in relation to follicle size. Develop Growth Differ. 2007;49(1):61–6.
Revelli A, Delle Piane L, Casano S, Molinari E, Massobrio M, Rinaudo P. Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod Biol Endocrinol. 2009;7(1):40.
Basuino L, Silveira JC. Human follicular fluid and effects on reproduction. JBRA assisted reproduction. 2016;20(1):38–40.
McRae C, Baskind NE, Orsi NM, Sharma V, Fisher J. Metabolic profiling of follicular fluid and plasma from natural cycle in vitro fertilization patients—a pilot study. Fertil Steril. 2012;98(6):1449–57.
Jayaraman V, Ghosh S, Sengupta A, Srivastava S, Sonawat HM, Narayan PK. Identification of biochemical differences between different forms of male infertility by nuclear magnetic resonance (NMR) spectroscopy. J Assist Reprod Genet. 2014;31(9):1195–204.
Lindon JC, Nicholson JK, Holmes E, Everett JR. Metabonomics: metabolic processes studied by NMR spectroscopy of biofluids. Concepts in Magnetic Resonance: An Educational Journal. 2000;12(5):289–320.
Kolokolova TN, Savel’ev OY, Sergeev NM. Metabolic analysis of human biological fluids by 1 H NMR spectroscopy. J Anal Chem. 2008;63(2):104.
Gowda GN, Raftery D. Can NMR solve some significant challenges in metabolomics? J Magn Reson. 2015;260:144–60.
Barding GA, Salditos R, Larive CK. Quantitative NMR for bioanalysis and metabolomics. Anal Bioanal Chem. 2012;404(4):1165–79.
Karaer A, Tuncay G, Mumcu A, Dogan B. Metabolomics analysis of follicular fluid in women with ovarian endometriosis undergoing in vitro fertilization. Syst Biol Reprod Med. 2019;65(1):39–47.
Atiomo W, Daykin CA. Metabolomic biomarkers in women with polycystic ovary syndrome: a pilot study. MHR: Basic science of reproductive medicine. 2012;18(11):546–53.
Hoffman JM, Lyu Y, Pletcher SD, Promislow DE. Proteomics and metabolomics in ageing research: from biomarkers to systems biology. Essays Biochem. 2017;61(3):379–88.
Piñero-Sagredo E, Nunes S, de los Santos MJ, Celda B, Esteve V. NMR metabolic profile of human follicular fluid. NMR Biomed 2010;23(5):485–495.
Fan TWM. Metabolite profiling by one-and two-dimensional NMR analysis of complex mixtures. Prog Nucl Magn Reson Spectrosc. 1996;28(2):161–219.
Hashemitabar M, Bahmanzadeh M, Mostafaie A, Orazizadeh M, Farimani M, Nikbakht R. A proteomic analysis of human follicular fluid: comparison between younger and older women with normal FSH levels. Int J Mol Sci. 2014;15(10):17518–40.
Cordeiro FB, Montani DA, Pilau EJ, Gozzo FC, Fraietta R, Turco EGL. Ovarian environment aging: follicular fluid lipidomic and related metabolic pathways. J Assist Reprod Genet. 2018;35(8):1385–93.
Sutton-McDowall ML, Gilchrist RB, Thompson JG. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction. 2010;139(4):685–95.
Cetica P, Pintos L, Dalvit G, Beconi M. Involvement of enzymes of amino acid metabolism and tricarboxylic acid cycle in bovine oocyte maturation in vitro. Reproduction. 2003;126(6):753–63.
Brackett BG, Zuelke KA. Analysis of factors involved in the in vitro production of bovine embryos. Theriogenology. 1993;39(1):43–64.
Alvarez GM, Casiró S, Gutnisky C, Dalvit GC, Sutton-McDowall ML, Thompson JG, et al. Implications of glycolytic and pentose phosphate pathways on the oxidative status and active mitochondria of the porcine oocyte during IVM. Theriogenology. 2016;86(9):2096–106.
Alvarez GM, Dalvit GC, Cetica PD. Influence of the cumulus and gonadotropins on the metabolic profile of porcine cumulus–oocyte complexes during in vitro maturation. Reprod Domest Anim. 2012;47(5):856–64.
Cetica PD, Pintos LN, Dalvit GC, Beconi MT. Effect of lactate dehydrogenase activity and isoenzyme localization in bovine oocytes and utilization of oxidative substrates on in vitro maturation. Theriogenology. 1999;51(3):541–50.
Monniaux D. Driving folliculogenesis by the oocyte-somatic cell dialog: lessons from genetic models. Theriogenology. 2016;86(1):41–53.
Boland NI, Humpherson PG, Leese HJ, Gosden RG. Pattern of lactate production and steroidogenesis during growth and maturation of mouse ovarian follicles in vitro. Biol Reprod. 1993;48(4):798–806.
Gull I, Geva E, Lerner-Geva L, Lessing JB, Wolman I, Amit A. Anaerobic glycolysis: the metabolism of the preovulatory human oocyte. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1999;85(2):225–8.
Harlow CR, Winston RML, Margara RA, Hillier SG. Gonadotrophic control of human granulosa cell glycolysis. Hum Reprod. 1987;2(8):649–53.
Sutton ML, Gilchrist RB, Thompson JG. Effects of in-vivo and in-vitro environments on the metabolism of the cumulus–oocyte complex and its influence on oocyte developmental capacity. Hum Reprod Update. 2003;9(1):35–48.
Leese JH, Lenton EA. Glucose and lactate in human follicular fluid: concentrations and interrelationships. Hum Reprod. 1990;5:915–9.
Pacella L, Zander-Fox DL, Armstrong DT, Lane M. Women with reduced ovarian reserve or advanced maternal age have an altered follicular environment. Fertil Steril. 2012;98(4):986–94.
Jana SK, Dutta M, Joshi M, Srivastava S, Chakravarty B, Chaudhury K. 1H NMR based targeted metabolite profiling for understanding the complex relationship connecting oxidative stress with endometriosis. Biomed Res Int. 2013;2013.
Shalgi R, Kraicer PF, Soferman N. Gases and electrolytes of human follicular fluid. Reproduction. 1972;28(3):335–40.
Dale B, Menezo Y, Cohen J, DiMatteo L, Wilding M. Intracellular pH regulation in the human oocyte. Human Reproduction (Oxford, England). 1998;13(4):964–70.
Broekmans FJ, Mol BW, Habbema JDF, te Velde ER. Performance of basal follicle-stimulating hormone in the prediction of poor ovarian response and failure to become pregnant after in vitro fertilization: a meta-analysis. Fertil Steril. 2003;79(5):1091–100.
Broekmans FJ, Kwee J, Hendriks DJ, Mol BW, Lambalk CB. A systematic review of tests predicting ovarian reserve and IVF outcome. Hum Reprod Update. 2006;12(6):685–718.
Jirge PR. Ovarian reserve tests. J Hum Reprod Sci. 2011;4(3):108–13.
Eyupoglu ND, Guzelce EC, Acikgoz A, Uyanik E, Bjørndal B, Berge RK, et al. Circulating gut microbiota metabolite trimethylamine N-oxide (TMAO) and oral contraceptive use in polycystic ovary syndrome. Clin Endocrinol. 2019;91(6):810–5.
Li P, Zhong C, Li S, Sun T, Huang H, Chen X, et al. Plasma concentration of trimethylamine-N-oxide and risk of gestational diabetes mellitus. Am J Clin Nutr. 2018;108(3):603–10.
Rexidamu M, Li H, Jin H, Huang J. Serum levels of trimethylamine-N-oxide in patients with ischemic stroke. Biosci Rep. 2019;39(6):BSR20190515.
Subramaniam S, Fletcher C. Trimethylamine N-oxide: breathe new life. Br J Pharmacol. 2018;175(8):1344–53.
Griffin LE, Djuric Z, Angiletta CJ, Mitchell CM, Baugh ME, Davy KP, et al. A Mediterranean diet does not alter plasma trimethylamine N-oxide concentrations in healthy adults at risk for colon cancer. Food Funct. 2019;10(4):2138–47.
Funding
This study was supported by the scientific research projects unit of Inonu University (Grant Number 2016/56).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All patients included in the study provided written informed consent, and the study protocol was permitted by the Clinical Research Ethics Committee (Number 2015/38).
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dogan, B., Karaer, A., Tuncay, G. et al. High-resolution 1H-NMR spectroscopy indicates variations in metabolomics profile of follicular fluid from women with advanced maternal age. J Assist Reprod Genet 37, 321–330 (2020). https://doi.org/10.1007/s10815-020-01693-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-020-01693-x