Abstract
Purpose
The aim of this study was to determine whether an interchromosomal effect (ICE) occurred in embryos obtained from reciprocal translocation (rcp) and Robertsonian translocation (RT) carriers who were following a preimplantation genetic diagnosis (PGD) with whole chromosome screening with an aCGH and SNP microarray. We also analyzed the chromosomal numerical abnormalities in embryos with aneuploidy in parental chromosomes that were not involved with a translocation and balanced in involved parental translocation chromosomes.
Methods
This retrospective study included 832 embryos obtained from rcp carriers and 382 embryos from RT carriers that were biopsied in 139 PGD cycles. The control group involved embryos obtained from age-matched patient karyotypes who were undergoing preimplantation genetic screening (PGS) with non-translocation, and 579 embryos were analyzed in the control group. A single blastomere at the cleavage stage or trophectoderm from a blastocyst was biopsied, and 24-chromosomal analysis with an aCGH/SNP microarray was conducted using the PGD/PGS protocols. Statistical analyses were implemented on the incidences of cumulative aneuploidy rates between the translocation carriers and the control group.
Results
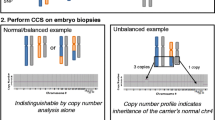
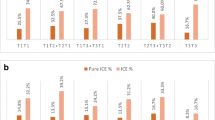
Reliable results were obtained from 138 couples, among whom only one patient was a balanced rcp or RT translocation carrier, undergoing PGD testing in our center from January 2012 to June 2014. For day 3 embryos, the aneuploidy rates were 50.7% for rcp carriers and 49.1% for RT carriers, compared with the control group, with 44.8% at a maternal age < 36 years. When the maternal age was ≥ 36 years, the aneuploidy rates were increased to 61.1% for rcp carriers, 56.7% for RT carriers, and 60.3% for the control group. There were no significant differences. In day 5 embryos, the aneuploidy rates were 24.5% for rcp carriers and 34.9% for RT carriers, compared with the control group with 53.6% at a maternal age < 36 years. When the maternal age was ≥ 36 years, the aneuploidy rates were 10.7% for rcp carriers, 26.3% for RT carriers, and 57.1% for the control group. The cumulative aneuploidy rates of chromosome translocation carriers were significantly lower than the control group. No ICE was observed in cleavage and blastocyst stage embryos obtained from these carriers. Additionally, the risk of chromosomal numerical abnormalities was observed in each of the 23 pairs of autosomes or sex chromosomes from day 3 and day 5 embryos.
Conclusion
There was not enough evidence to prove that ICE was present in embryos derived from both rcp and RT translocation carriers, regardless of the maternal age. However, chromosomal numerical abnormalities were noticed in 23 pairs of autosomes and sex chromosomes in parental structurally normal chromosomes. Thus, 24-chromosomal analysis with an aCGH/SNP microarray PGD protocol is required to decrease the risks of failure to diagnose aneuploidy in structurally normal chromosomes.



Similar content being viewed by others
References
Lledo B, Ortiz JA, Morales R, Ten J, de la Fuente PE, Garcia-Ochoa C, et al. The paternal effect of chromosome translocation carriers observed from meiotic segregation in embryos. Hum Reprod. 2010;25:1843–8.
Faraut T, Mermet MA, Demongeot J, Cohen O. Cooperation of selection and meiotic mechanisms in the production of imbalances in reciprocal translocations. Cytogenet Cell Genet. 2000;88:15–21.
Lejeune J. Autosomal disorders. Pediatrics. 1963;32:326–37.
Estop AM, Cieply K, Munne S, Surti U, Wakim A, Feingold E. Is there an interchromosomal effect in reciprocal translocation carriers? Sperm fish studies. Hum Genet. 2000;106:517–24.
Munne S. Analysis of chromosome segregation during preimplantation genetic diagnosis in both male and female translocation heterozygotes. Cytogenet Genome Res. 2005;111:305–9.
Alfarawati S, Fragouli E, Colls P, Wells D. Embryos of Robertsonian translocation carriers exhibit a mitotic interchromosomal effect that enhances genetic instability during early development. PLoS Genet. 2012;8:e1003025.
Gianaroli L, Magli MC, Ferraretti AP, Munne S, Balicchia B, Escudero T, et al. Possible interchromosomal effect in embryos generated by gametes from translocation carriers. Hum Reprod. 2002;17:3201–7.
Vozdova M, Oracova E, Musilova P, Kasikova K, Prinosilova P, Gaillyova R, et al. Sperm and embryo analysis of similar t(7;10) translocations transmitted in two families. Fertil Steril. 2011;96:e66–70.
Tan YQ, Tan K, Zhang SP, Gong F, Cheng DH, Xiong B, et al. Single-nucleotide polymorphism microarray-based preimplantation genetic diagnosis is likely to improve the clinical outcome for translocation carriers. Hum Reprod. 2013;28:2581–92.
Xie Y, Xu Y, Miao B, Zeng Y, Zhou C. A preliminary study on the application of array comparative genomic hybridization for preimplantaion genetic diagnosis. Chin J Med Genet. 2013;30:283–7.
Debrock S, Melotte C, Spiessens C, Peeraer K, Vanneste E, Meeuwis L, et al. Preimplantation genetic screening for aneuploid of embryos after in vitro fertilization in women aged at least 35 years: a prospective randomized trial. Fertil Steril. 2010;93:364–73.
Harton GL, Magli MC, Lundin K, Montag M, Lemmen J, Harper JC. Eshre pgd consortium/embryology special interest group—best practice guidelines for polar body and embryo biopsy for preimplantation genetic diagnosis/screening (pgd/pgs). Hum Reprod. 2011;26:41–6.
Xu YW, Zhou CQ, Zeng YH, Liu Y, Gao L, Zhuang GL. Clinical analysis of 100 preimplantation genetic diagnosis cycles. Chin J Obstet Gynecol. 2011;46:255–9.
Gardner DK, Lane M, Schoolcraft WB. Physiology and culture of the human blastocyst. J Reprod Immunol. 2002;55:85–100.
Tobler KJ, Brezina PR, Benner AT, Du L, Xu X, Kearns WG. Two different microarray technologies for preimplantation genetic diagnosis and screening, due to reciprocal translocation imbalances, demonstrate equivalent euploidy and clinical pregnancy rates. J Assist Reprod Genet. 2014;31:843–50.
Kirkpatrick G, Ma S. Meiotic segregation and interchromosomal effects in a rare (1:2:10) complex chromosomal rearrangement. J Assist Reprod Genet. 2012;29:77–81.
Kasikova K, Vozdova M, Prinosilova P, Gaillyova R, Hanakova M, Rubes J. Sperm meiotic segregation, aneuploid and high risk of delivering an affected offspring in carriers of non-Robertsonian translocation t(13;15). J Assist Reprod Genet. 2012;29:693–8.
Kovaleva NV. Increased risk of trisomy 21 in offspring of carriers of balanced non-contributing autosomal rearrangements is not explained by interchromosomal effect. Genetika. 2013;49:259–68.
Piomboni P, Stendardi A, Gambera L. Chromosomal aberrations and aneuploid of spermatozoa. Adv Exp Med Biol. 2014;791:27–52.
Vozdova M, Oracova E, Horinova V, Rubes J. Sperm fluorescence in situ hybridization study of meiotic segregation and an interchromosomal effect in carriers of t(11;18). Hum Reprod. 2008;23:581–8.
Juchniuk DVM, Santos SA, Pereira CS, Cuzzi JF, Laureano LA, Franco JJ, et al. Meiotic segregation and interchromosomal effect in the sperm of a double translocation carrier: a case report. Mol Cytogenet. 2009;2:24.
Bonnet-Garnier A, Guardia S, Pinton A, Ducos A, Yerle M. Analysis using sperm-fish of a putative interchromosomal effect in boars carrying reciprocal translocations. Cytogenet Genome Res. 2009;126:194–201.
Anton E, Vidal F, Blanco J. Reciprocal translocations: tracing their meiotic behavior. Genet Med. 2008;10:730–8.
Anton E, Vidal F, Blanco J. Interchromosomal effect analyses by sperm fish: incidence and distribution among reorganization carriers. Syst Biol Reprod Med. 2011;57:268–78.
Anton E, Blanco J, Vidal F. Meiotic behavior of three D;G Robertsonian translocations: segregation and interchromosomal effect. J Hum Genet. 2010;55:541–5.
Alfarawati S, Fragouli E, Colls P, Wells D. First births after preimplantation genetic diagnosis of structural chromosome abnormalities using comparative genomic hybridization and microarray analysis. Hum Reprod. 2011;26:1560–74.
Fiorentino F, Spizzichino L, Bono S, Biricik A, Kokkali G, Rienzi L, et al. Pgd for reciprocal and Robertsonian translocations using array comparative genomic hybridization. Hum Reprod. 2011;26:1925–35.
Pujol A, Durban M, Benet J, Boiso I, Calafell JM, Egozcue J, et al. Multiple aneuploid in the oocytes of balanced translocation carriers: a preimplantation genetic diagnosis study using first polar body. Reproduction. 2003;126:701–11.
Munne S, Escudero T, Fischer J, Chen S, Hill J, Stelling JR, et al. Negligible interchromosomal effect in embryos of Robertsonian translocation carriers. Reprod BioMed Online. 2005;10:363–9.
Tulay P, Gultomruk M, Findikli N, Yagmur E, Bahceci M. Is the interchromosomal effect present in embryos derived from Robertsonian and reciprocal translocation carriers particularly focusing on chromosome 10 rearrangements? Zygote. 2015;23:908–15.
Majumdar G, Majumdar A, Verma IC, Upadhyaya KC. Relationship between morphology, euploidy and implantation potential of cleavage and blastocyst stage embryos. J Hum Reprod Sci. 2017;10:49–57.
Minasi MG, Fiorentino F, Ruberti A, Biricik A, Cursio E, Cotroneo E, Varricchio MT, Surdo M, Spinella F, Greco E: Genetic diseases and aneuploid can be detected with a single blastocyst biopsy: a successful clinical approach. Hum Reprod. 2017;8:1770–77.
Wapner RJ. Genetics of stillbirth. Clin Obstet Gynaecol. 2010;53:628.
Cukurcam S, Sun F, Betzendahl I, et al. Trichlorfon predisposes to aneuploidy and interferes with spindle formation in in vitro maturing mouse oocytes. Mutat Res. 2004;2:165–78.
Flatters M, Maxfield R, Dawson D. The effects of a ring chromosome on the meiotic segregation of other chromosomes in Saccharomyces cerevisiae. Mol Gen Genet. 1995;3:309–316.
Yanxin X, Yanwen X, Benyu M, Yanhong Z, Jing W, Canquan Z. Clinical investigation to compare acgh and fish in preimplantationgenetic diagnosis of chromosome translocation carriers. Chin J Obstet Gynecol. 2014;49:193–8.
Spriggs EL, Rademaker AW, Martin RH. Aneuploid in human sperm: results of two-and three-color fluorescence in situ hybridization using centromeric probes for chromosomes 1, 12, 15, 18, x, and y. Cytogenet Cell Genet. 1995;71:47–53.
Blanco J, Egozcue J, Vidal F. Incidence of chromosome 21 disomy in human spermatozoa as determined by fluorescent in-situ hybridization. Hum Reprod. 1996;11:722–6.
Pellestor F, Girardet A, Coignet L, Andreo B, Charlieu JP. Assessment of aneuploid for chromosomes 8, 9, 13, 16, and 21 in human sperm by using primed in situ labeling technique. Am J Hum Genet. 1996;58:797–802.
Acknowledgements
The Nature and Science Foundation of Guangdong Province (S2012010009176); the Guangzhou City Science and Technology Board Foundation: the establishment of genetic disease preimplantation embryos and early pregnancy screening model (201300000097).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xie, Y., Xu, Y., Wang, J. et al. Preliminary analysis of numerical chromosome abnormalities in reciprocal and Robertsonian translocation preimplantation genetic diagnosis cases with 24-chromosomal analysis with an aCGH/SNP microarray. J Assist Reprod Genet 35, 177–186 (2018). https://doi.org/10.1007/s10815-017-1045-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-017-1045-9