Abstract
Purpose
The purpose of the present study is to study the relationship between oxidative stress (OS) in semen, semen characteristics, and reproductive outcomes in oocyte donation intracytoplasmic sperm injection (ICSI) cycles.
Methods
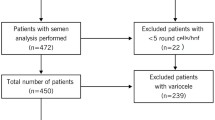
OS was measured in 132 semen samples.
Results
OS levels were as follows: very high (1.5 %), high (43.2 %), low (30.3 %), and very low (25.0 %). Overall seminal parameters were as follows: volume (ml) = 4.2 (SD 2.1), concentration (millions/ml) = 61.6 (SD 59.8), motility (a+b%) = 47.4 (SD 18.0), and normal spermatozoa (%) = 8.2 (SD 5.1). Of the 101 cycles that reached embryo transfer, 55.4 % evolved in biochemical, 46.5 % in clinical, and 43.6 % in ongoing pregnancy. OS level does not relate to seminal parameters, fertilization rate, or pregnancy outcomes.
Conclusions
OS testing by nitro blue tetrazolium (NBT) in fresh ejaculate might not be useful for all patients. Reproductive results with young oocytes and ICSI do not seem to be affected by OS-level semen.
Similar content being viewed by others
References
Wright C, Milne S, Leeson H. Sperm DNA damage caused by oxidative stress: modifiable clinical, lifestyle and nutritional factors in male infertility. Reprod Biomed Online. 2014;28(6):684–703. doi:10.1016/j.rbmo.2014.02.004.
Aitken RJ, Clarkson JS. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil. 1987;81(2):459–69.
Alvarez JG, Touchstone JC, Blasco L, Storey BT. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa. Superoxide dismutase as major enzyme protectant against oxygen toxicity. J Androl. 1987;8(5):338–48.
de Lamirande E, O’Flaherty C. Sperm activation: role of reactive oxygen species and kinases. Biochim Biophys Acta. 2008;1784(1):106–15. doi:10.1016/j.bbapap.2007.08.024.
Aitken RJ, Paterson M, Fisher H, Buckingham DW, van Duin M. Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J Cell Sci. 1995;108(Pt 5):2017–25.
Oehninger S, Blackmore P, Mahony M, Hodgen G. Effects of hydrogen peroxide on human spermatozoa. J Assist Reprod Genet. 1995;12(1):41–7.
Tremellen K. Oxidative stress and male infertility—a clinical perspective. Hum Reprod Update. 2008;14(3):243–58. doi:10.1093/humupd/dmn004.
Shamsi MB, Kumar R, Bhatt A, Bamezai RN, Kumar R, Gupta NP, et al. Mitochondrial DNA mutations in etiopathogenesis of male infertility. Indian J Urol : IJU : J Urol Soc India. 2008;24(2):150–4.
Aitken RJ, De Iuliis GN. On the possible origins of DNA damage in human spermatozoa. Mol Hum Reprod. 2010;16(1):3–13. doi:10.1093/molehr/gap059.
Ribas-Maynou J, Garcia-Peiro A, Fernandez-Encinas A, Amengual MJ, Prada E, Cortes P, et al. Double stranded sperm DNA breaks, measured by Comet assay, are associated with unexplained recurrent miscarriage in couples without a female factor. PLoS One. 2012;7(9):e44679. doi:10.1371/journal.pone.0044679.
Leduc F, Nkoma GB, Boissonneault G. Spermiogenesis and DNA repair: a possible etiology of human infertility and genetic disorders. Syst Biol Reprod Med. 2008;54(1):3–10. doi:10.1080/19396360701876823.
Marcon L, Boissonneault G. Transient DNA strand breaks during mouse and human spermiogenesis new insights in stage specificity and link to chromatin remodeling. Biol Reprod. 2004;70(4):910–8. doi:10.1095/biolreprod.103.022541.
Ribas-Maynou J, Fernandez-Encinas A, Garcia-Peiro A, Prada E, Abad C, Amengual MJ, et al. Human semen cryopreservation: a sperm DNA fragmentation study with alkaline and neutral Comet assay. Andrology. 2014;2(1):83–7. doi:10.1111/j.2047-2927.2013.00158.x.
Marchetti F, Essers J, Kanaar R, Wyrobek AJ. Disruption of maternal DNA repair increases sperm-derived chromosomal aberrations. Proc Natl Acad Sci U S A. 2007;104(45):17725–9. doi:10.1073/pnas.0705257104.
Gonzalez-Marin C, Gosalvez J, Roy R. Types, causes, detection and repair of DNA fragmentation in animal and human sperm cells. Int J Mol Sci. 2012;13(11):14026–52. doi:10.3390/ijms131114026.
Noblanc A, Damon-Soubeyrand C, Karrich B, Henry-Berger J, Cadet R, Saez F, et al. DNA oxidative damage in mammalian spermatozoa: where and why is the male nucleus affected? Free Radic Biol Med. 2013;65:719–23. doi:10.1016/j.freeradbiomed.2013.07.044.
Hammoud SS, Nix DA, Zhang H, Purwar J, Carrell DT, Cairns BR. Distinctive chromatin in human sperm packages genes for embryo development. Nature. 2009;460(7254):473–8. doi:10.1038/nature08162.
Hammadeh ME, Al Hasani S, Rosenbaum P, Schmidt W, Fischer Hammadeh C. Reactive oxygen species, total antioxidant concentration of seminal plasma and their effect on sperm parameters and outcome of IVF/ICSI patients. Arch Gynecol Obstet. 2008;277(6):515–26. doi:10.1007/s00404-007-0507-1.
Yeung CH, De Geyter C, De Geyter M, Nieschlag E. Production of reactive oxygen species by and hydrogen peroxide scavenging activity of spermatozoa in an IVF program. J Assist Reprod Genet. 1996;13(6):495–500.
Zorn B, Vidmar G, Meden-Vrtovec H. Seminal reactive oxygen species as predictors of fertilization, embryo quality and pregnancy rates after conventional in vitro fertilization and intracytoplasmic sperm injection. Int J Androl. 2003;26(5):279–85.
WHO. Laboratory manual for the examination and processing of human semen. 5th ed. Geneva: World Health Organization; 2010. 2010.
Coroleu B, Barri PN, Carreras O, Belil I, Buxaderas R, Veiga A, et al. Effect of using an echogenic catheter for ultrasound-guided embryo transfer in an IVF programme: a prospective, randomized, controlled study. Hum Reprod. 2006;21(7):1809–15. doi:10.1093/humrep/del045.
Tunc O, Thompson J, Tremellen K. Development of the NBT assay as a marker of sperm oxidative stress. Int J Androl. 2010;33(1):13–21. doi:10.1111/j.1365-2605.2008.00941.x.
Morielli T, O’Flaherty C. Oxidative stress impairs function and increases redox protein modifications in human spermatozoa. Reproduction. 2015;149(1):113–23. doi:10.1530/REP-14-0240.
Hosseinzadeh Colagar A, Karimi F, Jorsaraei SG. Correlation of sperm parameters with semen lipid peroxidation and total antioxidants levels in astheno- and oligoasheno-teratospermic men. Iran Red Crescent Med J. 2013;15(9):780–5. doi:10.5812/ircmj.6409.
Agarwal A, Tvrda E, Sharma R. Relationship amongst teratozoospermia, seminal oxidative stress and male infertility. Reprod Biol Endocrinol : RB&E. 2014;12:45. doi:10.1186/1477-7827-12-45.
Aitken RJ, Finnie JM, Muscio L, Whiting S, Connaughton HS, Kuczera L, et al. Potential importance of transition metals in the induction of DNA damage by sperm preparation media. Hum Reprod. 2014;29(10):2136–47. doi:10.1093/humrep/deu204.
Badouard C, Menezo Y, Panteix G, Ravanat JL, Douki T, Cadet J, et al. Determination of new types of DNA lesions in human sperm. Zygote. 2008;16(1):9–13. doi:10.1017/S0967199407004340.
Ozmen B, Koutlaki N, Youssry M, Diedrich K, Al-Hasani S. DNA damage of human spermatozoa in assisted reproduction: origins, diagnosis, impacts and safety. Reprod Biomed Online. 2007;14(3):384–95.
Aitken RJ, Gordon E, Harkiss D, Twigg JP, Milne P, Jennings Z, et al. Relative impact of oxidative stress on the functional competence and genomic integrity of human spermatozoa. Biol Reprod. 1998;59(5):1037–46.
Gandini L, Lombardo F, Paoli D, Caruso F, Eleuteri P, Leter G, et al. Full-term pregnancies achieved with ICSI despite high levels of sperm chromatin damage. Hum Reprod. 2004;19(6):1409–17. doi:10.1093/humrep/deh233.
Sakkas D, Alvarez JG. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril. 2010;93(4):1027–36. doi:10.1016/j.fertnstert.2009.10.046.
Seli E, Gardner DK, Schoolcraft WB, Moffatt O, Sakkas D. Extent of nuclear DNA damage in ejaculated spermatozoa impacts on blastocyst development after in vitro fertilization. Fertil Steril. 2004;82(2):378–83. doi:10.1016/j.fertnstert.2003.12.039.
Meseguer M, Martinez-Conejero JA, O’Connor JE, Pellicer A, Remohi J, Garrido N. The significance of sperm DNA oxidation in embryo development and reproductive outcome in an oocyte donation program: a new model to study a male infertility prognostic factor. Fertil Steril. 2008;89(5):1191–9. doi:10.1016/j.fertnstert.2007.05.005.
Loft S, Kold-Jensen T, Hjollund NH, Giwercman A, Gyllemborg J, Ernst E, et al. Oxidative DNA damage in human sperm influences time to pregnancy. Hum Reprod. 2003;18(6):1265–72.
Esbert M, Pacheco A, Vidal F, Florensa M, Riqueros M, Ballesteros A, et al. Impact of sperm DNA fragmentation on the outcome of IVF with own or donated oocytes. Reprod Biomed Online. 2011;23(6):704–10. doi:10.1016/j.rbmo.2011.07.010.
Ko EY, Sabanegh Jr ES, Agarwal A. Male infertility testing: reactive oxygen species and antioxidant capacity. Fertil Steril. 2014;102(6):1518–27. doi:10.1016/j.fertnstert.2014.10.020.
Author information
Authors and Affiliations
Corresponding author
Additional information
Capsule
Semen oxidative stress was measured in 132 patients’ ejaculates by nitro blue tetrazolium testing before performing ICSI with donor oocytes. The measured level did not correlate with either seminal parameters or reproductive outcomes.
Rights and permissions
About this article
Cite this article
Pujol, A., Obradors, A., Esteo, E. et al. Oxidative stress level in fresh ejaculate is not related to semen parameters or to pregnancy rates in cycles with donor oocytes. J Assist Reprod Genet 33, 529–534 (2016). https://doi.org/10.1007/s10815-016-0660-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10815-016-0660-1