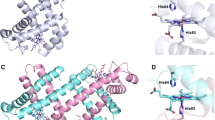
Molecular oxygen (O2) migration in isolated β-chains of human hemoglobin was studied using molecular dynamics simulations and laser kinetic absorption spectroscopy. Insertion of xenon (Xe) atoms into the isolated chains was found to decrease the time constant of the slowest component of geminal O2 rebinding to the protein. This change was caused by a decrease in the intra-protein space available for O2 migration after insertion of the inert gas into the Xe-binding sites of the protein. Molecular dynamics simulations revealed that the O2 molecule occupied both the Xe2 and Xe1 sites of the protein during geminal recombination to the isolated β-chains. The amino acids involved in formation of the primary and secondary docking sites of the protein were determined. The results are important for understanding the mechanism of O2 binding by both native tetrameric human hemoglobin and artificial oxygen carriers based on heme proteins.
Similar content being viewed by others
References
E. Antonini and M. Brunori, Hemoglobin and Myoglobin in their Reactions with Ligands, North-Holland Publication Company, Amsterdam, London (1971).
M. F. Perutz, Nature, 228, 726–739 (1970).
Q. H. Gibson, Biochem. J., 71, 293–303 (1959).
J. S. Philo and J. W. Lary, J. Biol. Chem., 265, 139–143 (1990).
I. Birukou, R. L. Schweers, and J. S. Olson, J. Biol. Chem., 285, 8840–8854 (2010).
S. V. Lepeshkevich, N. V. Konovalova, and B. M. Dzhagarov, Biochemistry (Moscow), 68, No. 5, 551–558 (2003).
B. M. Dzhagarov and S. V. Lepeshkevich, Chem. Phys. Lett., 390, Nos. 1–3, 59–64 (2004).
S. V. Lepeshkevich, N. V. Konovalova, I. I. Stepuro, and B. M. Dzhagarov, J. Mol. Struct., 735–736, 307–313 (2005).
S. V. Lepeshkevich and B. M. Dzhagarov, FEBS J., 272, No. 23, 6109–6119 (2005).
D. A. Duddell, R. J. Morris, N. J. Muttucumaru, and J. T. Richards, Photochem. Photobiol., 31, 479–484 (1980).
R. J. Morris, Q. H. Gibson, M. Ikeda-Saito, and T. Yonetani, J. Biol. Chem., 259, 6701–6703 (1984).
D. A. Chernoff , R. M. Hochstrasser, and A. W. Steele, Proc. Natl. Acad. Sci. USA, 77, 5606–5610 (1980).
B. M. Dzhagarov, V. A. Galievsky, N. N. Kruk, and M. D. Yakutovich, Dokl. Akad. Nauk, 366, 38–41 (1999).
S. V. Lepeshkevich, J. Karpiuk, I. V. Sazanovich, and B. M. Dzhagarov, Biochemistry, 43, No. 6, 1675–1684 (2004).
Q. H. Gibson, Biochemistry, 38, No. 16, 5191–5199 (1999).
W. A. Eaton, L. K. Hanson, P. J. Stephens, J. C. Sutherland, and J. B. R. Dunn, J. Am. Chem. Soc., 100, No. 16, 4991–5003 (1978).
B. I. Greene, R. M. Hochstrasser, R. B. Weisman, and W. A. Eaton, Proc. Natl. Acad. Sci. USA, 75, No. 11, 5255–5259 (1978).
S. V. Lepeshkevich, I. V. Sazanovich, M. V. Parkhats, S. N. Gilevich, and B. M. Dzhagarov, Chem. Sci., 12, No. 20, 7033–7047 (2021).
A. Pesce, M. Nardini, S. Dewilde, L. Capece, M. A. Marti, S. Congia, M. D. Salter, G. C. Blouin, D. A. Estrin, P. Ascenzi, L. Moens, M. Bolognesi, and J. S. Olson, J. Biol. Chem., 286, 5347–5358 (2011).
B. A. Springer, S. G. Sligar, J. S. Olson, and G. N. Phillips, Jr., Chem. Rev., 94, 699–714 (1994).
J. M. Friedman, Science, 228, 1273–1280 (1985).
C. Savino, A. E. Miele, F. Draghi, K. A. Johnson, G. Sciara, M. Brunori, and B. Vallone, Biopolymers, 91, No. 12, 1097–1107 (2009).
S. V. Lepeshkevich, S. A. Biziuk, A. M. Lemeza, and B. M. Dzhagarov, Biochim. Biophys. Acta, 1814, No. 10, 1279–1288 (2011).
M. F. Lucas and V. Guallar, Biophys. J., 102, 887–896 (2012).
M. S. Shadrina, G. H. Peslherbe, and A. M. English, Biochemistry, 54, 5268–5278 (2015).
M. S. Shadrina, A. M. English, and G. H. Peslherbe, J. Am. Chem. Soc., 134, 11177–11184 (2012).
M. Takayanagi, I. Kurisaki, and M. Nagaoka, J. Phys. Chem. B, 117, 6082–6091 (2013).
S. V. Lepeshkevich, S. N. Gilevich, M. V. Parkhats, and B. M. Dzhagarov, Biochim. Biophys. Acta, 1864, No. 9, 1110–1121 (2016).
E. Bucci and C. Fronticelli, J. Biol. Chem., 240, 551–552 (1965).
R. C. Neuman, Jr., W. Kauzmann, and A. Zipp, J. Phys. Chem., 77, 2687–2691 (1973).
S. V. Lepeshkevich and B. M. Dzhagarov, Biochim. Biophys. Acta, 1794, 103–109 (2009).
S. V. Lepeshkevich, M. V. Parkhats, A. S. Stasheuski, V. V. Britikov, E. S. Jarnikova, S. A. Usanov, and B. M. Dzhagarov, J. Phys. Chem. A, 118, No. 10, 1864–1878 (2014).
S. V. Lepeshkevich, A. L. Poznyak, and B. M. Dzhagarov, J. Appl. Spectrosc., 72, No. 5, 735–743 (2005).
S. V. Lepeshkevich, M. V. Parkhats, I. I. Stepuro, and B. M. Dzhagarov, Biochim. Biophys. Acta, 1794, No. 12, 1823–1830 (2009).
H. J. C. Berendsen, D. Van der Spoel, and R. Van Drunen, Comput. Phys. Commun., 91, 43–56 (1995).
D. Van der Spoel, E. Lindahl, B. Hess, G. Groenhof, A. E. Mark, and H. J. C. Berendsen, J. Comput. Chem., 26, 1701–1719 (2005).
E. Lindahl, B. Hess, and D. Van der Spoel, J. Mol. Model., 7, 306–317 (2001).
W. Humphrey, A. Dalke, and K. Schulten, J. Mol. Graphics, 14, 33–38 (1996).
Y. Cheng, T.-J. Shen, V. Simplaceanu, and C. Ho, Biochemistry, 41, 11901–11913 (2002).
I. Birukou, J. Soman, and J. S. Olson, J. Biol. Chem., 286, 10515–10529 (2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Prikladnoi Spektroskopii, Vol. 90, No. 3, pp. 361–369, May-June, 2023.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lepeshkevich, S.V., Parkhats, M.V., Biziuk, S.A. et al. Molecular Oxygen Migration in Isolated β-Chains of Human Hemoglobin as Revealed by Molecular Dynamics Simulations and Laser Kinetic Spectroscopy. J Appl Spectrosc 90, 485–492 (2023). https://doi.org/10.1007/s10812-023-01557-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-023-01557-z