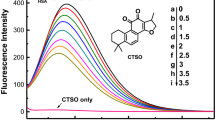
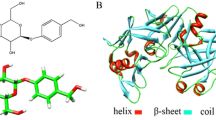
Site-selective binding between pioglitazone hydrochloride (PGH) and trypsin (TRP) was investigated by fluorescence spectroscopy, ultraviolet spectroscopy, synchronous fluorescence spectroscopy, and circular dichroism spectroscopy. The results demonstrated that PGH could quench the intrinsic fluorescence of TRP strongly by a static quenching process. The microenvironment of tryptophan (Trp) and tyrosine (Tyr) residue was both changed, and the polarity of the hydrophobic environment in the TRP cavity was enhanced and the hydrophobicity was weakened. The results demonstrated that the interaction between PGH and TRP was taking place via hydrogen bond and hydrophobic force with 1:1 binding ratio. The binding constants Ka, the number of binding sites n, and thermodynamic parameters were obtained. The participation of amino acid residues and synchronous spectroscopy showed that the main site of the reaction between PGH and TRP was at the hydrophobic cavity. The binding rate of PGH to protein in 298 K was ~65–85% and for the combined model was W = –0.08684R2 + 0.1048R + 0.8503. This provides the theoretical basis for the dosage of the drug.
Similar content being viewed by others
References
C. C. Zhang and A. J. Matzger, Cryst. Growth. Des., 17, No. 2, 414–417 (2017).
T. Takagi, C. Ramachandran, M. Bermejo, S. Yamashita, L. X. Yu, and G. L. Amidon, Mol. Pharm., 3, No. 6, 631–643 (2006).
Y. Y. Liu, G. W. Zhang, Y. J. Liao, and Y. P. Wang, Spectrochim. Acta A, 151, 498–505 (2015).
X. X. Hu, Z. H. Yu, and R. T. Liu, Spectrochim. Acta A, 108, 50–54 (2013).
R. Y. Wang, X. H. Wang, R. Q. Wang, R. Wang, H. J. Dou, J. Wu, C. J. Song, and J. B. Chang, J. Lumin., 138, 258–266 (2013).
Y. Y. Liu, G. W. Zhang, N. Zeng, and S. Hu, Spectrochim. Acta A, 173, 188–195 (2017).
W. Liu, J. P. Liu, L. Q. Zou, Z. Q. Zhang, C. M. Liu, R. H. Liang, M. Y. Xie, and J. Wan, Food Chem., 146, 278–283 (2014).
K. K. Ding, H. X. Zhang, H. F. Wang, X. Lv, L. M. Pan, W. J. Zhang, and S. L. Zhang, J. Hazard. Mater., 299, 486–494 (2015).
Q. Wang, J. W. He, J. Yan, D. Wu, and H. Li, J. Lumin., 30, No. 2, 240–246 (2015).
Y. Z. Zhang, X. X. Chen, A. Dai, X. P. Zhang, Y. X. Liu, and Y. Liu, J. Lumin., 23, No. 3, 150–156 (2008).
J. H. Shi, K. L. Zhou, Y. Y. Lou, and D. Q. Pan, Spectrochim. Acta A, 188, 362–371 (2018).
M. Toprak and M. Arik, Luminescence, 29, No. 7, 805–809 (2014).
J. E. Ali and P. A. Vahid, Food Chem., 202, 426–431 (2016).
Z. Y. Tian, F. L. Zang, W. Luo, Z. H. Zhao, Y. G. Wang, and C. J. Wang, J. Photochem. Photobiol. B, 142, 103–109 (2015).
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Zhurnal Prikladnoi Spektroskopii, Vol. 86, No. 2, pp. 235–240, March–April, 2019.
Rights and permissions
About this article
Cite this article
Wang, CD., Liu, BS., Bian, G. et al. Spectroscopic Study of Site-Selective Binding of Pioglitazone Hydrochloride to Trypsin. J Appl Spectrosc 86, 250–255 (2019). https://doi.org/10.1007/s10812-019-00808-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-019-00808-2