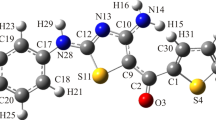
Vibrational IR (3200–650 cm–1) and Raman spectra (3200–150 cm–1) of adamantane-containing 3-(adamantan-1-yl)-4-ethyl-1-[(4-phenylpiperazin-1-yl)methyl]-1H-1,2,4-triazole-5(4H)-thione, which is promising for drug design, were examined. The UV/Vis spectrum (450–200 nm) of the compound in EtOH was measured. Full geometry optimization using density functional theory (DFT) in the B3LYP/cc-pVDZ approximation allowed the equilibrium configuration of the molecule to be determined and IR and Raman spectra to be calculated. Based on these, the experimental vibrational IR and Raman spectra were interpreted and the biological activity indices were predicted. The UV/Vis spectrum of the title compound was simulated at the time-dependent DFT/CAM-B3LYP/cc-pVDZ level with and without solvent effects and at the ab initio multi-reference perturbation theory XMCQDPT2 level. The UV/Vis spectrum that was simulated using the multi-reference XMCQDPT2 approximation agreed very successfully with the experimental data, in contrast to the single-reference DFT method. This was probably a consequence of intramolecular charge transfer.
Similar content being viewed by others
References
G. Lamoureux and G. Artavia, Curr. Med. Chem., 17, 2967–2978 (2010).
J. Liu, D. Obando, V. Liao, T. Lifa, and R. Codd, Eur. J. Med. Chem., 46, 1949–1963 (2011).
G. Ali Mansoori, P. L. Barros de Araujo, and E. Silvano de Araujo, Diamondoid Molecules: With Applications in Biomedicine, Materials Science, Nanotechnology & Petroleum Science, World Scientific Publishing (2012).
F. G. Hayden, Antiviral Res., 71, 372–378 (2006).
M.-J. Perez-Perez, J. Balzarini, M. Hosoya, E. De Clercq, and M.-J. Camarasa, Bioorg. Med. Chem. Lett., 2, 647–648 (1992).
I. Stylianakis, A. Kolocouris, N. Kolocouris, G. Fytas, G. B. Foscolos, E. Padalko, J. Neyts, and E. De Clercq, Bioorg. Med. Chem. Lett., 13, 1699–1703 (2003).
N. A. Ilyushina, N. V. Bovin, R. G. Webster, and E. A. Govorkova, Antiviral Res., 70, 121–131 (2006).
G. Zoidis, C. Fytas, I. Papanastasiou, G.B. Foscolos, G. Fytas, E. Padalko, E. De Clercq, L. Naesens, J. Neyts, and N. Kolocouris, Bioorg. Med. Chem., 14, 3341–3348 (2006)
A. A. El-Emam, O. A. Al-Deeb, M. A. Al-Omar, and J. Lehmann, Bioorg. Med. Chem., 12, 5107–5113 (2004).
M. Protopopova, C. Hanrahan, B. Nikonenko, R. Samala, P. Chen, J. Gearhart, L. Einck, and C. A. Nacy, J. Antimicrob. Chemother., 56, 968–974 (2005).
A. A. El-Emam, A.-M. S. Al-Tamimi, M. A. Al-Omar, K. A. Alrashood, and E. E. Habib, Eur. J. Med. Chem., 68, 96–102 (2013).
A. A. Kadi, E. S. Al-Abdullah, I. A. Shehata, E. E. Habib, T. M. Ibrahim, and A. A. El-Emam, Eur. J. Med. Chem., 45, 5006–5011 (2010).
K. Omar, A. Geronikaki, P. Zoumpoulakis, C. Camoutsis, M. Sokovic, A. Ciric, and J. Glamoclija, Bioorg. Med. Chem., 18, 426–432 (2010).
A. A. Kadi, N. R. El-Brollosy, O. A. Al-Deeb, E. E. Habib, T. M. Ibrahim, and A. A. El-Emam, Eur. J. Med. Chem., 42, 235–242 (2007).
E. S. Al-Abdullah, H. H. Asiri, S. Lahsasni, E. E. Habib, T. M. Ibrahim, and A. A. El-Emam, Drug Des., Dev. Ther., 8, 505–518 (2014).
O. Kouatly, A. Geronikaki, C. Kamoutsis, D. Hadjipavlou-Litina, and P. Eleftheriou, Eur. J. Med. Chem., 44, 1198–1204 (2009).
O. A. Al-Deeb, M. A. Al-Omar, N. R. El-Brollosy, E. E. Habib, T. M. Ibrahim, and A. A. El-Emam, Arzneim. Forsch./Drug Res., 56, 40–47 (2006).
S. Riganas, I. Papanastasiou, G. B. Foscolos, A. Tsotinis, J.-J. Bourguignon, G. Serin, J.-F. Mirjolet, K. Dimas, V. N. Kourafalos, A. Eleutheriades, V. I. Moutsos, H. Khan, S. Georgakopoulou, A. Zaniou, M. Prassa, M. Theodoropoulou, S. Pondiki, and A. Vamvakides, Bioorg. Med. Chem., 20, 3323–3331 (2012).
A. A. El-Emam, E. S. Al-Abdullah, H. M. Al-Tuwaijri, M. Said-Abdelbaky, and S. Garcia-Granda, Acta Crystallogr., Sect. E: Struct. Rep. Online, 68, o2380–o2381 (2012).
M. B. Shundalau, E. S. Al-Abdullah, E. V. Shabunya-Klyachkovskaya, A. V. Hlinisty, O. A. Al-Deeb, A. A. El-Emam, and S. V. Gaponenko, J. Mol. Struct., 1115, 258–266 (2016).
A. M. Andrianov, I. A. Kashyn, V. M. Andrianov, M. B. Shundalau, A. V. Hlinisty, S. V. Gaponenko, E. V. Shabunya-Klyachkovskaya, A. Matsukovich, A.-M. S. Al-Tamimi, and A. A. El-Emam, J. Chem. Sci., 128, 1933–1942 (2016).
M. Shundalau, Y. Mindarava, A. Matsukovich, S. Gaponenko, and A. A. El-Emam, in: Abstracts of the XIVth International Conference on Molecular Spectroscopy (ICMS2017), Bialka Tatrzanska, Poland (2017), p. 136.
M. W. Schmidt, K. K. Baldridge, J. A. Boatz, S. T. Elbert, M. S. Gordon, J. H. Jensen, S. Koseki, N. Matsunaga, K. A. Nguyen, S. J. Su, T. L. Windus, M. Dupuis, and J. A. Montgomery, Jr., J. Comput. Chem., 14, 1347–1363 (1993).
B. M. Bode and M. S. Gordon, J. Mol. Graphics Modell., 16, 133–138 (1998).
L. J. Farrugia, J. Appl. Crystallogr., 30, 565 (1997).
T. H. Dunning, Jr., J. Chem. Phys., 90, 1007–1023 (1989).
A. D. Becke, J. Chem. Phys., 98, 5648–5652 (1993).
C. Lee, W. Yang, and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 37, 785–789 (1988).
P. J. Stephens, F. J. Devlin, C. F. Chabalowski, and M. J. Frisch, J. Phys. Chem., 98, 11623–11627 (1994).
E. Runge and E. K. U. Gross, Phys. Rev. Lett., 52, 997–1000 (1984).
K. Burke, J. Werschnik, and E. K. U. Gross, J. Chem. Phys., 123, 062206 (2005).
T. Yanai, D. P. Tew, and N. C. Handy, Chem. Phys. Lett., 393, 51–57 (2004).
A. V. Marenich, C. J. Cramer, and D. G. Truhlar, J. Phys. Chem. B, 113, 6378–6396 (2009).
A. A. Granovsky, J. Chem. Phys., 134, 214113 (2011).
Alex A. Granovsky, Firefl y version 8; http://classic.chem.msu.su/gran/fi refly/index.html.
A. Lagunin, A. Stepanchikova, D. Filimonov, and V. Poroikov, Bioinformatics, 16, 747–748 (2000).
PASS Online; http://way2drug.com/passonline/index.php.
G. A. Pitsevich, M. B. Shundalau, M. A. Ksenofontov, and D. S. Umreiko, Global J. Anal. Chem., 2, 114–124 (2011).
V. Arjunan, T. Rani, C. V. Mythili, and S. Mohan, Spectrochim. Acta, Part A, 79, 486–496 (2011).
I. Hargittai and K. Hedberg, in: Molecular Structures and Vibrations, S. J. Cyvin (Ed.), Elsevier Publishing Co., New York (1972), pp. 340–357.
N. W. Larsen, J. Mol. Struct., 51, 175–190 (1979).
S. Gunasekaran and B. Anita, Indian J. Pure Appl. Phys., 46, 833–838 (2008).
K. Kuchitsu (Ed.), Structure of Free Polyatomic Molecules. Basic Data, Springer (1998).
L. Bistricic, G. Baranovic, and K. Mlinaric-Majerski, Spectrochim. Acta, Part A, 51, 1643–1664 (1995).
E. I. Bagrii, Adamantanes: Preparation, Properties, Applications [in Russian], Nauka, Moscow (1989).
R. M. Silverstein, F. X. Webster, and D. J. Kiemle, Spectrometric Identification of Organic Compounds, 7th edn., Wiley & Sons, Inc., (2005).
L. Goodman, A. G. Ozkabak, and S. N. Thakur, J. Phys. Chem., 95, 9044–9058 (1991).
P. J. Hendra and D. B. Powell, Spectrochim. Acta, 18, 299–306 (1962).
O. Alver, C. Parlak, and M. Şenyel, Spectrochim. Acta, Part A, 67, 793–801 (2007).
S. A. Kudchadker and C. N. R. Rao, Indian J. Chem., 11, 140–142 (1973).
F. Billes, H. Endredi, and G. Keresztury, J. Mol. Struct.: THEOCHEM, 530, 183–200 (2000).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Prikladnoi Spektroskopii, Vol. 85, No. 2, pp. 181–193, March–April, 2018.
Rights and permissions
About this article
Cite this article
Mindarava, Y.L., Shundalau, M.B., Al-Wahaibi, L.H. et al. Spectral Analysis of 3-(Adamantan-1-yl)-4-Ethyl-1-[(4-Phenylpiperazin-1-yl) Methyl]-1H-1,2,4-Triazole-5(4H)-Thione. J Appl Spectrosc 85, 203–215 (2018). https://doi.org/10.1007/s10812-018-0633-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10812-018-0633-5