Abstract
Volatile fatty acids (VFA) and reducing sugars (RS) are widely used as platform molecules in biorefineries, facilitating the production of valuable biofuels and chemicals. From an environmental, economic and social perspective, third generation biomass, including macroalgae beach-cast, represents an innovative and optimal solution for the production of these commodities. This study explores the impact of ultrasound pretreatment on the invasive macroalga Rugulopteryx okamurae, aiming to produce RS and VFA through enzymatic hydrolysis and dark fermentation. Several ultrasound conditions were tested: amplitudes (0, 70-100 %), suspension volumes (300, 600 mL), and algal concentrations (4-8 %). Optimal results emerged with 100 % amplitude, 300 mL volume, and 4 % (w/v) algal concentration, leading to the maximum COD solubilization of 61.5 mg COD g-biomass-1. For enzymatic hydrolysis, the pretreated sample achieved maximum RS concentrations (0.124 g-RS g-biomass-1) with half the enzyme dosage required by the non-pretreated alga (25 vs 50 FPU g-biomass-1), implying significant economic benefits for large-scale processes. The kinetic model proposed by Romero-Vargas et al. aligned perfectly with the experimental data, obtaining higher values of all the kinetic parameters for the pretreated sample. Dark fermentation showed substantial increases in organic matter solubilization and VFA production (10.36 mg-HAc g-biomass-1) post ultrasound pretreatment: 21.1 % higher solubilization and 9.4 % increased VFA compared to non-pretreated biomass. The resulting VFA composition comprised 73 % acetic acid, 13 % propionic acid, and 8 % butyric acid. Utilization of chemical agents during sonication may further enhance overall processing yields.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seaweeds or macroalgae are macroscopic marine algae (Pereira 2021; Saleem et al. 2024) and are marine photosynthetic organisms (Farghali et al. 2023). They are categorized into green, red and brown algae based on their photosynthetic pigments (Aboudi et al. 2021). They play a crucial role in the balance of the marine ecosystem, regulating ocean acidification, mitigating eutrophication and enhancing carbon sequestration. Algae provide an adequate habitat for the ecosystem (Farghali et al. 2023). However, there is great concern about the invasion of seaweed into non-native waters, as it poses a threat to coastal ecosystems and their habitats. In Europe, for instance, seaweed invasions represent more than 40 % of exotic marine invasive species (Roca et al. 2022).
Rugulopteryx okamurae, belonging to the Dictyotaceae (Barcellos et al. 2023), is a brown macroalga identified as an invasive species in Spain (García-Gómez et al. 2020). This alga originates from the Western Pacific Ocean (Estévez et al. 2022). In 2002, it was accidentally introduced in the Mediterranean Sea in France. About thirteen years later, it was found in Ceuta (García-Gómez et al. 2020; Estévez et al. 2022), marking its first appearance on the coast of the Strait of Gibraltar. By 2017, its geographical distribution was deemed unusual compared to previous invasion cases, as it had colonized more than 65 % of the rock surfaces in a single year (García-Gómez et al. 2020). Since then, it has rapidly expanded along the Andalusian coastline, stretching from the Gulf of Cádiz to Málaga (Estévez et al. 2022). Nowadays, this alga attains maximum coverage levels (90 %), disrupting the proper functioning of the ecosystem in the coastal habitats of Cádiz (Florido et al. 2023). It accumulates in substantial quantities and has displaced essential components of the native marine flora, resulting in the loss of ecosystem services and resources (Altamirano et al. 2019; García-Gómez et al. 2020). Furthermore, its prolific growth has adverse repercussions on various sectors of the local economy, including fishing and tourism (Altamirano et al. 2019).
The valorization of algal biomass offers an environmentally friendly alternative to landfill disposal, enabling its use as a raw material for various other processes. Macroalgae serve as a valuable third-generation bioresource, yielding a variety of products, including pigments, lipids, proteins, polysaccharides and their monosaccharides, volatile fatty acids (VFA), biostimulants, and materials suitable for composting (Fernández-Medina et al. 2022; Arias et al. 2023; Barcellos et al. 2023; Pardilhó et al. 2023). Monosaccharides and VFA, in particular, are crucial precursors within the modern biorefinery concept, offering a diverse range of industrial applications, such as bioplastics and biofuels (Lara et al. 2020; Sekoai et al. 2021; Agabo-García et al. 2023). Enzymatic hydrolysis and dark fermentation are two key bioprocesses employed to obtain monosaccharides and VFA, respectively (de la Lama-Calvente et al. 2021).
Enzymatic hydrolysis, also referred to as enzymatic saccharification, is a process mediated by enzymes in which the fermentable sugars constituting biomass polysaccharides are released into the medium (Lara et al. 2020). Enzymatic hydrolysis is environmentally friendly, generating no toxic residues, and is a relatively low-consuming energy process since it is typically conducted under mild pressure and thermal conditions (Ribeiro et al. 2019).
On the other hand, the dark fermentation process is part of the overall anaerobic digestion process and is also known as acidogenic anaerobic digestion. It involves biological oxidation conducted by a complex microbiota in the absence of molecular oxygen. This process comprises two stages: hydrolysis, where organic macromolecules are broken down into monomers, and acidogenesis, where these monomers are transformed into VFA, CO2, and H2 (Kumar et al. 2019; Saratale et al. 2021; Sekoai et al. 2021; Kim et al. 2022).
Due to the complexity of the macroalgae structure, accessibility to organic matter is restricted, limiting its use as a substrate for biological processes, such as enzymatic hydrolysis or dark fermentation (Thompson et al. 2019). Several pretreatment methods have shown promising potential in enhancing the organic matter solubilization from algal biomass, including mechanical, microwave, ultrasound, thermal, chemical, and biological pretreatments (Azizi et al. 2017; Thompson et al. 2019; Fernández-Medina et al. 2022; Agabo-García et al. 2023; Romero-Vargas et al. 2023a, b).
Some authors have studied enzymatic hydrolysis and dark fermentation processes applied to R. okamurae both with and without previous pretreatments. For instance, microwave irradiation (Fernández-Medina et al. 2022), microwave-assisted hydrothermal (Ferreira-Anta et al. 2023), biological (Agabo-García et al. 2023) and hydrothermal acid (Azizi et al. 2017; Romero-Vargas et al. 2023a) have been tested. Thus, Fernández-Medina et al. (2022) investigated the effect of microwave radiation on organic matter solubilization in this alga and subsequently conducted enzymatic hydrolysis or dark fermentation processes. They achieved chemical oxygen demand (COD) values ranging from 2 to 13.5 g O2 L-1 after algal pretreatment. In the enzymatic hydrolysis and the dark fermentation processes, the highest data for reducing sugars (RS) and VFA were 160 mg-RS g-biomass-1 and 46.3 mg-VFA g-biomass-1, respectively (Fernández-Medina et al. 2022). In a different study, Romero-Vargas et al. (2023a) assessed the effect of hydrothermal and hydrothermal acid pretreatments on enzymatic hydrolysis of the alga R. okamurae. In this research, RS production values ranged from 12.47 to 24.67 g L-1 depending on the acid concentrations. Similarly, Agabo-García et al. (2023) investigated the effect of biological pretreatment with the fungus Aspergillus awamori in solid-state fermentation on saccharification. This process enabled the production of approximately 240 mg-RS g-biomass-1.
Ultrasound pretreatment involves the application of rapid cycles of compression and depression of sonic waves to the biomass. This process, also known as sonification, creates air cavities or microbubbles within the cell wall. When these cavities collapse, they disrupt the cellular wall, leading to changes in the morphology of the substrate (Thompson et al. 2019; Dobrinčić et al. 2020; Cui and Zhu 2021; Sidana and Yadav 2022). Furthermore, this process helps the generation of VFA more easily later (Passos et al. 2015; Thompson et al. 2019).
Chemat et al. (2017) attribute the effectiveness of this pretreatment method to the combined effects of mixing with the physical impacts of ultrasound on the raw material. They identified several mechanisms that act during ultrasound-assisted extraction, including erosion, local shear stress, fragmentation, sonoporation, sonocapillary effect and destruction-detexturation of structures. Moreover, ultrasound pretreatment enhances the mass transfer rate due to the intense mixing effect created by the propagation of ultrasound in the liquid medium. At a macroscopic scale, this mixing effect is a result of acoustic streaming, while acoustic microstreaming occurs at a local level.
As previously mentioned, in the compression cycle the bubbles collapse and, therefore, temporary hot spots are produced resulting in heat energy dissipation and the consequent elevation of the temperature of the medium (Flint and Suslick 1991; Chemat et al. 2017). Previous studies (Karray et al. 2015; Chemat et al. 2017; Garcia-Vaquero et al. 2018; Yi et al. 2020) have demonstrated that ultrasound treatment is highly effective when conducted within the temperature range of 20 to 80 ºC. However, at lower temperatures (Chemat et al. 2017), cavitation-induced bubbles tend to be smaller and collapse with greater intensity, thereby inducing alterations in cell wall integrity and mass transfer. Higher temperatures can influence cavitation effects, as they promote the easier generation of bubbles but reduce their collapse intensity due to damping effects (Yi et al. 2020; Cui and Zhu 2021; Rayo-Mendez et al. 2021).
The composition and amount of organic matter released during ultrasound pretreatment varies with sonication amplitude, pretreatment time (Sidana and Yadav 2022), temperature, solvent (Cui and Zhu 2021), algal concentration in the medium (Romero-Vargas et al. 2023b) and the volume to be sonicated. In a recent study by Romero-Vargas et al. (2023b), ultrasound pretreatment of the brown alga Dictyota dichotoma and the subsequent enzymatic hydrolysis were analyzed. These authors tested two biomass concentrations (2.1 and 4.3 % w/v), different amplitude percentages and various pretreatment times to improve the saccharification of algal biomass. The highest production of reducing sugars was achieved with an algal concentration of 4.3 % w/v, 40 min pretreatment, and a 40 % amplitude setting .
The objective of the present study is to investigate the effect of ultrasound pretreatment on the seaweed R. okamurae for subsequent bioprocessing in enzymatic hydrolysis or dark fermentation, with the aim of obtaining RS and VFA, respectively. VFA and sugars are widely used as platform molecules in a wide range of industries for the production of commercially valuable biofuels and chemicals. Indeed, there are nowadays two platforms, namely sugar and carboxylates, that could be potentially used for the partial replacement of the petrochemical industry.
The utilization of macroalgal biomass represents a competitive advantage over the use of other types of biomass (first or second generation) as it does not compete with food and land use and contains practically no lignin. Furthermore, in the case of the invasive seaweed R. okamurae, it constitutes a waste that must be managed as such to avoid its accumulation in coastal areas, given the environmental, economic and social problems it generates. To the best of our knowledge, this study has not been carried out previously.
Materials and methods
Figure 1 shows a scheme of the algal bioprocessing performed in this study.
Raw material and conditioning
Rugulopteryx okamurae was obtained from algae deposits at Los Lances Beach, in Tarifa (Cádiz, Spain) in October 2021. The seaweed was conditioned after collection (washing, drying and grinding of the alga, with a particle size distribution that ranged between 0.25 and 2 mm).
Ultrasound pretreatment
The algal biomass was pretreated with a UP400S Heilscher Ultrasound Technology Ultrasonicator, with an output power of 400 W. The H14 TIP 14 probe used has an acoustic density of 105 W cm-2, a maximum amplitude of 125 μm with 14 mm diameter and a maximum submersion depth of 90 mm. In addition, the equipment has a jacketed stainless steel vessel that allows temperature regulation of the sample. A Selecta Digiterm TFT P thermostat-cryostat bath was coupled to this jacketed stainless steel vessel.
During the pretreatment, the temperature of the thermostat-cryostat bath was established at 10 °C, to avoid a high increase in the suspension temperature during the ultrasonic pulses. It is known that operating at low temperatures can favor sonochemical effects and temperature control is usually applied to limit their increase (Meireles et al. 2014; Chemat et al. 2017). The inside vessel temperature was measured with a Hanna model HI 935005N probe thermometer inserted in the ultrasound vessel. All ultrasound pretreatment experiments were performed for 40 min (Romero-Vargas et al. 2023b) and at a constant pulse of 0.5. For ultrasound pretreatment studies, all suspensions were prepared with deionized water.
Selection of ultrasound pretreatment parameters
The influence of the percentage of ultrasound amplitude and the volume of the algal suspension to be pretreated on the COD was studied. The ultrasound amplitude was tested at 0, 70, 80, 90 and 100 %, where 0 % represents the non-pretreated algae. Prior studies on brown algae investigated ultrasound amplitudes up to 60 % (Romero-Vargas et al. 2023b), revealing the positive impact of amplitude increments. Hence, in this study, we opted to extend the amplitude percentages beyond these values. Additionally, the volumes of the algal suspensions used were 300 and 600 mL. The 300 mL volume represents the minimum requirement of the sonotrode, while the 600 mL volume was tested to assess the effect of increasing the suspension volume on the pretreatment. Throughout these experiments, the algal concentration remained fixed at 4 % (w/v).
Additionally, the impact of the alga concentration on the COD was analyzed for concentrations of 4, 6 and 8 % (w/v). These tests were conducted at the best conditions determined in the previous studies about the percentage of ultrasound amplitude and the suspension volume.
Each experimental condition was conducted in triplicate, selecting as most adequate those obtaining the higher COD values. Once the best pretreatment conditions were determined, a sufficient amount of algal suspension was pretreated by ultrasonication for its characterization by quantifying the COD, Total polyphenols (TP), VFA and RS, as well as performing the subsequent bioprocessing.
Enzyme hydrolysis and dark fermentations assays
As known, the product yields from the enzymatic hydrolysis and dark fermentation processes, namely reducing sugars and volatile fatty acids, respectively, increase when the ultrasonication pretreatment efficiency increases (Shankaran et al. 2022; Snehya et al. 2022; Romero-Vargas et al. 2023b; Shabarish et al. 2023). Therefore, enzymatic hydrolysis and dark fermentation tests were carried out with the ultrasonicated sample of algal suspension.
Enzyme hydrolysis assays
After pretreatment of the algae with ultrasound, the suspension was centrifuged. Since previous studies observed lower yields in enzymatic hydrolysis when maintaining the total suspension (Fernández-Medina et al. 2022), the supernatant was discarded, preserving only the solids. The sample was centrifuged for 10 min at 2820 ×g. The wet biomass was spread on filter paper and dried in a Royal convection tray drying oven model RCDA-1350/100S at 40 ºC for 24 h.
For enzymatic hydrolysis, 4.5 g of the pretreated and dried algal biomass were weighed into a 100 mL Erlenmeyer flask. Then 45 mL of a phosphate buffer solution (0.05 mol L-1 and pH 5) was added to each flask. The concentration of pretreated alga in the hydrolysis was 10 % (w/v). This value was fixed by considering previous studies that showed that this alga concentration allowed us to obtain the highest RS concentration (Romero-Vargas et al. 2023b). Then, the flasks were sterilized in a Selecta Autoclave model Autotester ST Dry PV III at 120 °C for 20 min. After the sterilization, 337 μL of Cellic CTec2 enzyme complex (Novozyme Corp.) (25 FPU g-biomass-1) was added to each flask in a laminar flow cabinet. The samples were maintained in an orbital incubator (IKA model KS 4000 i control) at 50 °C and 250 rpm for 72 h. Samples of 500 μL were taken off at different hydrolysis times (0; 0.5; 1; 1.5; 2; 2.5; 3; 4; 5; 6; 8; 24; 30; 48 and 72 h) for RS concentration measurement. All tests were conducted in triplicate.
Additionally, several assays were conducted with non-pretreated seaweed to analyze the effect of ultrasonic pretreatment on enzymatic hydrolysis. The impact of agitation rate was studied using 150 and 250 rpm and the effect of enzyme dosage was explored with 30 and 50 FPU g-biomass-1.
The experimental data were fitted to the kinetic model for enzymatic hydrolysis proposed by Romero-Vargas et al. (2023c). This model incorporates a term considering conventional first-order hydrolysis kinetics and an additional term to account for enzyme diffusion and reaction within the solid particle.
The expression of the model is given by
where: P is the RS concentration (g L-1) at time t, P0 is the initial RS concentration (g L-1), β is the hydrolysis yield coefficient, k1 is the rate constant (h-1), t is the hydrolysis time (h) and So is the initial substrate concentration that can be converted to product (g L-1), which was obtained from the total dietary fibre without lignin contained in the seaweed suspension (27.29 g L-1), α is the hydrolysis yield coefficient for the enzyme diffusion stage and k2 is the rate constant (g L-1 h-1) for this stage.
Dark fermentation assays
For the dark fermentation tests the following samples were prepared: the ultrasound pretreated alga, the non-pretreated alga and an inoculum control. The inoculum used came from the effluent of an acidogenic reactor, operated in semicontinuous mode at a temperature of 55 °C (thermophilic range) and with a hydraulic retention time of 6 days. This acidogenic reactor was fed with exhausted sugar beet pulp, supplied by AB Sugar, a sugar factory located in Jerez de la Frontera (Cádiz, Spain). To obtain the inoculum, 600 mL of effluent was centrifuged at 3500 rpm for 10 min, removing the solids and using the supernatant as inoculum. The ultrasound pretreated alga and the non-pretreated alga were mixed with the inoculum in a proportion of 90 % alga and 10 % inoculum. The inoculum control consisted of 100 % inoculum.
The pH of all samples was adjusted to 7.5 with sodium hydroxide (0.1 mol L-1). The dark fermentation tests were carried out in amber bottles with a total volume of 250 mL. 135 mL of the medium (sample and/or inoculum) described above was added to each bottle. The rest of the volume of the bottle was the chamber for produced biogas accumulation. All the bottles were covered and oxygen was removed by passing a nitrogen flux. They were subsequently incubated at 55 ºC in a Selecta Incudigit oven. These trials were maintained for 13 days.
To determine the composition of the gases, samples were taken off at 1, 2, 3, 5, 7, 9 and 13 days. Both the gas pressure in the bottles and the ambient pressure were measured daily using a digital manometer (Omega HPP350). From the above data on pressures and compositions of the gas generated, the volume of hydrogen produced in the dark fermentation process was determined. The values of pH, COD, VFA and TP content were measured at the beginning and the end of the dark fermentation process.
All the dark fermentation tests were conducted in triplicate.
Analytical determinations
The quantification of RS was carried out using the DNS spectrophotometric method adapted to microplates (Gonçalves et al. 2010). This is an adaptation of the DNS colorimetric method. COD and pH analyses were performed according to the standardized methods (Bridgewater 2017) of the American Public Health Association (APHA). The TP quantifications were performed according to the Folin-Ciocalteu method (Espada-Bellido et al. 2017).
The biogas composition generated in the dark fermentation process was analyzed using a Shimadzu gas chromatograph model GC‐2014. This equipment has a thermal conductivity detector (TCD) and a Carbosieve S-II model packed column of 2 m in length and 3.2 mm in diameter. The detector operated at 225 °C and the column initially at a temperature of 100 °C for 1 min, applying a ramp of 20 °C min-1 until reaching 190 °C, a temperature that remained constant for 2 min. The carrier gas was nitrogen at a flow of 50 mL min-1. The total duration of the method is 7.5 min.
Short-chain VFA concentrations were measured on a GC‐2010 gas chromatograph with a flame ionization detector (FID) (Shimadzu), equipped with an AOC‐20i autoinjector (Shimadzu). The equipment has a capillary column filled with Nukol (Supelco), 0.25 mm in diameter, 30 m long and a 0.25 μm packing film. Hydrogen was used as the carrier gas and detector fuel (50 mL min-1), synthetic air was used as oxidizing gas (400 mL min-1), and nitrogen was used as auxiliary (makeup) gas (30 mL min-1).
The total VFA concentration was determined as the weighted average of the individual VFA concentrations, obtained in the chromatograph, based on their molecular weights and expressed as acetic acid (HAc).
Specific productivity
The specific productivities of the enzymatic hydrolysis and dark fermentation tests were determined as the final value of each parameter per gram of alga used. Thus, specific productivities of the RS, VFA, COD and TP were calculated after pretreatment with ultrasound. All assays were performed in triplicate, and the mean value of the results was calculated. Additionally, the confidence limit was determined in each case, calculated according to Student's t for a confidence level of 95 %. Data are expressed as mean ± confidence limit.
Finally, a multivariable experimental design (five levels for amplitude and two levels for volume of algal suspension and using three replicates) was performed to select the best pretreatment conditions. Commercial Minitab software (Minitab 16 Statistical Software) was used for statistical analysis and experimental design, with COD as the response variable.
Results
Ultrasound pretreatment
During the ultrasonic pretreatment of R. okamurae, an elevation in the internal vessel temperature was detected. Thus, as a result of the ultrasonic pulses, the temperature reached 25 ± 5 ºC. A temperature increase of 1.5 and 3 times the initial value was observed. Therefore, it is worth noting that the temperature reached in the vessel for all the assays is appropriate for the study carried out in this work.
In order to determine the optimal pretreatment operating conditions, an analysis was conducted on the effects of varying the percentage of ultrasound amplitude, the volume of the algae suspension, and the algal concentration on the COD solubilization. COD serves as a measure of the efficiency and extent of sample disintegration resulting from ultrasound pretreatment. A higher disintegration degree implies greater effectiveness in solubilizing organic matter.
Effect of percentage of amplitude and algae suspension volume on COD solubilization
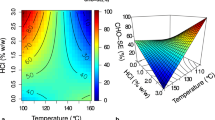
Initially, an investigation was carried out to assess the impact of the operating variables, i.e., the percentage of ultrasound amplitude and the volume of the alga suspension to be pretreated (Figure 2a). These assays were performed by using an algal concentration of 4 % (w/v). The results demonstrated a COD increase ranging from 41 % to 203 % compared to non-pretreated algae samples. When a 300 mL volume of alga suspension was employed, the chemical oxygen demand showed a positive correlation with the ultrasound amplitude.
Similar observations were made when varying the amplitude from 70 to 90 % at a volume of 600 mL of alga suspension. However, a deviation from this trend occurred when increasing the amplitude to 100 %, where a slight decrease in COD value was observed.
To assess the statistical significance of the studied variables in COD solubilization, an experimental design was executed. The percentage of ultrasound amplitude was analyzed across 5 levels (0, 70, 80, 90, 100 %), with 0 % indicating non-pretreated algae. Variation in the volume of algae suspension was explored at 2 levels (300 and 600 mL). The results revealed that both factors and their interaction had a statistically significant influence on COD at a 95 % confidence level. The response surface graph depicted a peak at the lower volume level (300 mL) and the highest amplitude tested (100 %) (Figure 2b).
Effect of algal concentration on COD solubilization
The influence of algal biomass concentration on pretreatment was investigated using the optimal conditions previously determined in the earlier study (100 % amplitude and a 300 mL volume). Figure 3 illustrates the COD concentrations achieved and specific data related to the quantity of algal biomass (specific productivities). As shown, the pretreatment of algal biomass consistently resulted in an increase in both the concentration of COD dissolved in the medium and the specific productivity values. When comparing the results of the pretreated samples, it is evident that maximum solubilization occurs at a 4 % concentration, with a more pronounced effect observed when comparing specific productivity values.
Characterization of algal biomass pretreated under selected conditions
Based on the optimal results obtained from the selection of ultrasound pretreatment parameters, a sufficient quantity of algal suspension was subjected to pretreatment for the subsequent studies. The chosen operating conditions were 100 % ultrasound amplitude, a 300 mL volume of the algae suspension, 40 min of pretreatment and 4 % (w/v) algal concentration. The concentrations of COD, TP, VFA, and RS were determined and are presented in Table 1. It should be noted that the only VFA generated during the pretreatment was acetic acid.
Enzymatic hydrolysis of algal biomass
A specific enzymatic hydrolysis assay was conducted to evaluate the productivity of RS from pretreated algal biomass at a concentration of 10 % (w/v). Additionally, several assays were performed with non-pretreated algal biomass to analyze the effect of pretreatment on subsequent enzymatic hydrolysis. Figure 4 presents the experimental RS data obtained from the hydrolysis of non-pretreated algae under various conditions (150 and 250 rpm and 30 and 50 FPU g-biomass-1), alongside data from the hydrolysis of ultrasound-pretreated algae. In this study, the maximum specific productivity of reducing sugars, relative to the amount of substrate used, reached 0.124 g-RS g-biomass-1 (12.4 g L-1). Additionally, the figure includes curve fits of these experimental data to the model proposed by Romero-Vargas et al. (2023c). The results of fitting the model to the experimental outcomes of different assays are detailed in Table 2. As observed, the considered model aligns perfectly with the experimental results.
The P0 value for the pretreated sample is lower, starting the pretreatment with a lower concentration of RS. However, the kinetic parameters (β, k1, α, and k2) were higher when the sample was pretreated with ultrasound, indicating that this pretreatment advantaged the hydrolysis.
As observed in Figure 4, the highest RS concentrations were achieved for the pretreated sample at the optimum conditions (12.4 g-RS L-1) and for the non-pretreated alga hydrolysed at 250 rpm and 25 FPU g-biomass-1 (12.2 g-RS L-1). Other conditions tested with non-pretreated algae (NP-150-30 and P-150-50) achieved significantly lower RS concentrations.
Dark fermentation assays
Dark fermentation experiments were conducted to evaluate the productivity of VFA and bio-hydrogen using ultrasound-pretreated algal biomass. In addition, non-pretreated algal biomass underwent fermentation to investigate the impact of pretreatment on subsequent VFA generation.
No methane formation was detected in the biogas from the assays carried out, which indicates the absence of methanogenic archaea in the inoculum. The final pH value of the samples was 5.83 ± 0.11 for the inoculum control; 6.33 ± 0.15 for the non-pretreated algae; and 6.16 ± 0.14 for the pretreated algae. All these values fall within the pH range considered suitable for the dark fermentation process.
Table 3 shows the values of COD, VFA, H2 and TP obtained for non-pretreated (NP) algal biomass and the following treatments applied to algal biomass: ultrasound pretreated (UP), dark fermentation (DF) and ultrasonic pretreatment followed by dark fermentation (UP+DF). Data for the dark fermentation process (DF and UP+DF) have been recalculated to subtract the inoculum contribution. The results showed an increase in the values of the pretreated algae compared to the non-pretreated algal biomass. Similar results were observed with the UP+DF process compared to the dark fermentation process with non-pretreated algal biomass.
Figure 5a illustrates the contributions of the inoculum and algal biomass to VFA at the beginning (t0) and the end (tf) of dark fermentation. Specific productivities in dark fermentation were 9.52 and 10.36 mg-HAc g-biomass-1 in non-pretreated and pretreated algal biomass, respectively (Figure 5b).
In the UP+DF overall process, the volatile fatty acid (VFA) composition consisted of 73 % acetic acid, 13 % propionic acid, and 8 % butyric acid, with the remaining volatile fatty acids being negligible. In contrast, for non-pretreated seaweed, the composition was 65 % acetic acid, 8 % propionic acid, and 17 % butyric acid (Figure 6).
The total hydrogen accumulation during the dark fermentation test was determined (Figure 7a), revealing a small volume of hydrogen produced yet demonstrating a notable positive impact of ultrasound pretreatment, resulting in a 56 % increase.
Similarly, Figure 7b illustrates the contribution of the inoculum, non-pretreated, and pretreated alga to the specific productivity of H2. The specific productivity of H2 for the non-pretreated algae was 0.51 mL H2 g-alga-1, while for the pretreated alga, it was 0.81 mL H2 g-alga-1.
Discussion
Effects of amplitude percentage, alga suspension volume and algal concentration on COD solubilization
In the studies performed with a volume of the algal suspension of 300 mL, it was observed that the COD solubilization increased with the amplitude, owing to the enhanced alga disintegration. It must be considered that the amplitude increment raises the intensity of pretreatment and sonication energy. Furthermore, an increase in amplitude resulted in a more vigorous bubble collapse, causing higher disruption upon implosion (Chemat et al. 2017). This observation aligns with findings reported by multiple authors in the context of ultrasound pretreatments (Shankaran et al. 2022; Snehya et al. 2022; Romero-Vargas et al. 2023b).
For amplitude increments from 70 to 80 % and 80 to 90 %, the change in COD concentration was marginal, with increases of only 7 % and 1.7 %, respectively. These findings suggest that there is not a substantial improvement in pretreatment efficiency. However, at an amplitude of 100 %, a significant increase in COD concentration till 2459 mg O2 mL-1 (57 % increase compared to 90 % amplitude) was observed. This significant increase in COD concentration at 100 % amplitude can be attributed to the amplification of energy received per unit area. At this amplitude, the bubbles implode with greater intensity, causing more pronounced cell ruptures and thus increasing the availability of organic matter. Similar observations were reported by Shankaran et al. (2022) in their study of marine macroalgae Chaetomorpha antennina (alga concentration 2 % w/v). They observed that within the low-intensity range, partial solubilization of the macroalga occurred. However, at higher amplitudes, a remarkable enhancement in the release of soluble organic compounds was noted. This improvement resulted from the combined influence of high-power ultrasonic waves, pressure waves, and increased bubble formation within the cavity, leading to cell wall rupture and solubilization of organic matter. Additionally, it is noteworthy that, in all cases, higher COD values were obtained when the sample volume to be pretreated was 300 mL compared to the 600 mL. This difference can be attributed to power per unit volume reduction as the sample volume increases, subsequently diminishing the ultrasound efficiency (Chemat et al. 2017; Snehya et al. 2022). In addition, the results observed in the response surface graph were found in accordance with the experimental findings. The maximum COD value was observed at 300 mL and 100 % amplitude.
Moreover, it was found that increasing the algal biomass concentration does not enhance the organic matter solubilization process, likely due to reduced biomass exposition to ultrasonic irradiation. Furthermore, higher biomass concentration leads to increased viscosity, which can further limit exposure (Youssouf et al. 2017; Park and Jeong 2021; Romero-Vargas et al. 2023b). In line with the findings of Youssouf et al. (2017), who evaluated ultrasound-assisted extraction of polysaccharides, the highest yields were achieved at the lowest alga/water ratio, attributed to improved diffusion owing to greater concentration differences between the intracellular and external medium. At higher biomass concentrations, this diffusion is impeded.
Characterization of algal biomass pretreated under selected conditions
The results obtained in the pretreatment (Table 1) were in the same order of magnitude as those found in the literature (Romero-Vargas et al. 2023b). Moreover, the TP concentration obtained was similar to the concentration found for the R. okamurae alga pretreated with microwaves (Fernández-Medina et al. 2022).
Regarding RS production from algal biomass, most literature studies have focused on green algae. For instance, Karray et al. (2015) achieved a RS concentration of 2.53 g L-1 when pretreating Ulva rigida with ultrasound at 20 ºC with algal concentrations ranging from 10 to 80 % (w/v). Similarly, Fernandes et al. (2022) obtained specific productivities of RS of 20.30 mg g-biomass-1 using a similar pretreatment method with U. rigida at 10 % (w/v) in distilled water without temperature control.
The results obtained from ultrasound pretreatment of brown alga Dictyota dichotoma fall within the range of 4.6 – 5.7 mg g-biomass-1 when using an algal biomass concentration of 4.3 % (w/v) and in the range of 8.6 – 8.9 mg g-biomass-1 when the concentration is reduced to 2.1 % (w/v) (Romero-Vargas et al. 2023b). These experiments were conducted with the same pretreatment duration as the present study (40 min) but with amplitude percentages ranging from 20 to 60 %. The results are of the same order of magnitude as those presented in this study for R. okamurae, confirming the trend already indicated in Section "Effect of algal concentration on COD solubilization". Therefore, operating with low alga concentrations in ultrasound is advantageous, as it can lead to higher RS yields per gram of alga.
The results obtained for COD concentration and specific productivity align with findings from existing literature. Snehya et al. (2021) reported values of 46 mg O2 g-alga-1 during the pretreatment of Ulva fasciata (Snehya et al. 2022). In another study by the same authors, values of 72 mg O2 g-alga-1 were achieved when pretreating the brown alga Sargassum tennerimum solely with ultrasound (Snehya et al. 2022).
On the other hand, for dark fermentation, it is convenient to maximize the solubilization of organic matter during pretreatments while minimizing the polyphenol content in the extract. Phenolic compounds in macroalgae have been reported to have adverse effects on mixed anaerobic cultures (Fernández-Medina et al. 2022). The TP specific productivity obtained was 11 times higher than that observed by Saravana et al. (2024) when the brown alga Ascophyllum nodosum was pretreated with ultrasound (0.099 mg g-biomass-1). However, the TP concentrations in the non-pretreated alga R. okamurae (Table 3) (0.88 mg g-biomass-1) were also 9 times higher . It is important to note that the differences are largely dependent on the macroalgae species and pretreatment conditions (Olatunji et al. 2024).
Nevertheless, the TP concentration released during ultrasound pretreatment was similar to that obtained by Fernández-Medina et al. (2022) when they subjected the same alga (R. okamurae) to microwave pretreatment at temperatures ranging from 120 to 180 ºC.
Enzymatic hydrolysis of algal biomass
The choice of a 10 % algal concentration was based on previous studies that demonstrated improved RS productivity with R. okamurae at this concentration (Romero-Vargas et al. 2023c). In that study, the primary hydrolysis variables (biomass loading, enzyme dose, and stirring rates) and the operation mode (fed-batch versus batch) were assessed to maximize the sugar concentration achievable in the enzymatic hydrolysis of non-pretreated algal biomass. Optimal conditions, yielding a maximum total reducing sugar concentration of 13.7 g L-1, were determined as follows: biomass loading of 10 % (w/v), 50 FPU g-biomass-1, 250 rpm, and operating in batch mode. Similarly, Azizi et al. (2017) employed a 10 % (w/v) concentration in the enzymatic hydrolysis of the brown alga Sargassum sp. and obtained positive results for RS concentrations. Other studies also support the use of higher algal concentrations, indicating that increasing the algal concentration up to 80 % (w/v) was favorable for the process (Karray et al. 2015).
Regarding the effect of the pretreatment on the RS production, similar concentrations were obtained for both the pretreated (UP-250-25) and the non-pretreated alga (NP-250-50). It supposes a much lower enzyme dose is required to attain the same final RS concentration when the algal suspension is pretreated. Indeed, the same RS concentration level can be achieved using half the enzyme dosage, which holds significant economic implications, as the cost of enzymes is often the bottleneck in the enzymatic hydrolysis application on an industrial scale (Lara et al. 2020). In any case, the experimental data indicate that the maximum RS concentration could increase for longer hydrolysis times. This effect is observed in all conducted assays. However, hydrolysis time is a parameter that also impacts the industrial feasibility of the process, so excessive increases are not desirable.
The values of kinetic parameters (β, k1, α, and k2) are higher in all cases for the assay conducted with the pretreated sample. This suggests that both the initial hydrolysis stage of material dissolved in the liquid medium and the enzyme diffusion stage within the solid are favoured by ultrasound pretreatment. The parameter P0 is related to the RS content after sterilizing the algal suspension in the autoclave and adding the enzyme to it. The data indicate that this parameter is directly related to the enzyme dose used.
In this study, the maximum specific productivity of reducing sugars, relative to the amount of substrate used, reached 0.124 g-RS g-biomass-1 (12.4 g L-1). This data is in the same order of magnitude as those reported in the literature. Thus, Liu and Wang (2014) obtained 0.148 ± 0.012 g-RS g-biomass-1 from Laminaria japonica pretreated with ultrasound. Borines et al. (2013) achieved productivities of 0.12 g-RS g-biomass-1 by an acid pretreatment and hydrolysis and using the brown algae Sargassum spp., with an enzyme concentration of 50 FPU g-biomass-1. Additionally, Saravanan et al. (2018) reported an RS content of 0.11 ± 0.016 g-RS g-biomass-1 for Sargassum sp. following acid pretreatment and hydrolysis with cellulase (53 FPU g-biomass-1) and pectinase (20 IU g-biomass-1).
Similarly, enzymatic hydrolysis of Dictyota dichotoma pretreated by ultrasound (Romero-Vargas et al. 2023b) at a concentration of 4.3 %, an amplitude percentage of 60 %, for 40 min led to RS values of 0.16 g-RS g-biomass-1; however, the enzyme dose used was more than four times greater. In other study, these authors reported specific productivities ranging between 0.12 and 0.24 g-RS g-biomass-1 after saccharification of R. okamurae alga with a hydrothermal acid pretreatment. In that scenario, the enzyme dosage used was twice as high as in the present study (Romero-Vargas et al. 2023a). Similarly, Agabo-García et al. (2023) investigated the biological pretreatment and subsequent enzymatic hydrolysis of R. okamurae. They achieved maximum RS specific productivities of 0.239 mg-RS g-biomass-1 using 344 μL of the enzyme cocktail Cellic CTec2®.
Although the specific RS productivity results for R. okamurae in the above mentioned studies was higher than in that obtained in this work, it should be noted that other pretreatments, different to ultrasound, were used and also the enzyme dose was much higher. Nevertheless, the cost of enzymes continues to pose the primary obstacle to achieving complete economic viability in the enzymatic hydrolysis process (Lara et al. 2020).
Certainly, some researchers enhance the outcomes of ultrasound pretreatment by incorporating a chemical agent during the sonication process. For instance, Park and Jeong (2021) investigated reducing sugar production in a brown alga using a physicochemical pretreatment involving ultrasound and acid pretreatment, followed by enzymatic hydrolysis. They observed higher yields of reducing sugars (75 %) when ultrasound was combined with sulfuric acid pretreatment. However, in the absence of acid during ultrasound pretreatment, the yields dropped below 40 % . Acidic conditions during pretreatment are effective for cell wall disruption (Naseem et al. 2024). This could potentially serve as a pathway to increase the RS yield achieved in this study
Dark fermentation assays
The data in Table 3 show an increase in COD values following ultrasound pretreatment of algal biomass. This elevation can be attributed to the impact of sonic waves on the biomass cell walls, facilitating the solubilization of organic matter into the liquid medium. Subsequent dark fermentation processes result in a further rise in COD for both non-pretreated and pretreated algal biomass. As expected, the COD for the effluent from the overall process (UP+DF) is the highest. Consequently, the effect of ultrasound pretreatment on organic matter solubilization after dark fermentation leads to a 21.1 % increase compared to the dark fermentation of non-pretreated algal biomass.
The majority of the observed increases in total volatile fatty acids (VFA) could be attributed to the dark fermentation process. Notably, no VFA were detected in the non-pretreated samples, and only a small amount was measured in the UP effluents. Consistent with the earlier observation on COD, ultrasound pretreatment resulted in an enhancement of VFA production by 8.7 %.
The slightly higher proportion of propionic acid in the pretreated algae is noteworthy (Figure 6). This could be advantageous if the goal is to utilize the generated VFA as a carbon source in bacterial bioplastic production (Fernández-Medina et al. 2022). Previous studies have highlighted that the composition of polyhydroxyalkanoates heavily relies on the VFA composition fed to the culture. It has been observed that a high concentration of propionic acid and valeric acid tends to yield a copolymer of P(3HB-co-3HV) (poly(3-hydroxybutyrate-co-3-hydroxyvalerate)), showcasing enhanced mechanical properties. This copolymer exhibits lower stiffness and brittleness, along with higher flexibility, tensile strength, and toughness (Zhang et al. 2019; Saratale et al. 2021).
On the other hand, no latency periods in hydrogen generation were observed at the beginning of the DF assay (Figure 7a), indicating the suitability and activity of the used inoculum for algal biomass degradation. Furthermore, as observed in Table 3, although the polyphenolic content exhibits an increase in the dark fermentation effluent following seaweed pretreatment, the values obtained are not deemed sufficiently high to hinder the fermentation process (Sharma and Melkania 2017; Sun et al. 2020; Fernández-Medina et al. 2022).
Within the initial three days, a higher rate of H2 production was noted, after which the production stabilized. A 79 % and 126 % increase in H2 production was observed in the non-pretreated alga and pretreated alga, respectively, compared to the inoculum control. The volume of H2 obtained with the inoculum control aligns with those reported by Fernández-Medina et al. (2022) in their study using R. okamurae. These authors observed hydrogen yields akin to those of non-pretreated algae, even after microwave pretreatment at 160 ºC and 180 ºC.
Nevertheless, the specific productivity results obtained (Figure 7b) were notably lower compared to previous studies (Liu et al. 2014; Fernández-Medina et al. 2022; Shankaran et al. 2022; Snehya et al. 2022). Thus, the specific productivity observed during the dark fermentation of R. okamurae was 28 times lower than those achieved by Liu and Wang (2014), who subjected L. japonica to ultrasound pretreatment at a concentration of 1 % (w/v). Conversely, it ranged between 9 and 20 times lower than the productivity reported by Yin et al. (2019), who applied prior microwave treatment to L. japonica at a concentration of 2.4 % (w/v). Additionally, the productivities documented by Fernández-Medina et al. (2022) were between 1.7 and 14 times higher than those observed in this study.
Conclusions
The algal biomass was subjected to ultrasound pretreatment to enhance the organic matter solubilization before biological bioprocessing (enzymatic hydrolysis and dark fermentation). Optimal ultrasound pretreatment conditions were 300 mL algal suspension volume, 4% (w/v), 100 % amplitude and 40 min, leading to the maximum COD solubilization of 61.5 mg COD g-biomass-1.
After enzymatic hydrolysis of the algal biomass, the highest RS concentrations were achieved for the pretreated sample at the optimum conditions (12.4 g-RS L-1) and for the non-pretreated alga (12.2 g-RS L-1) but using double enzyme dosage. Therefore, the maximum specific productivity reached 0.124 g-RS g-biomass-1. This result holds significant economic implications, as the cost of enzymes is often the bottleneck in the enzymatic hydrolysis application on an industrial scale.
The kinetic model by Romero-Vargas et al. (2023c) for enzymatic hydrolysis fits adequately with experimental results. The values of kinetic parameters (β, k1, α, and k2) are higher in all cases for the assay conducted with the pretreated sample. This suggests that both the initial hydrolysis stage of material dissolved in the liquid medium and the enzyme diffusion stage within the solid are favoured by ultrasound pretreatment.
Regarding the dark fermentation process, the effect of ultrasound pretreatment on organic matter solubilization after dark fermentation leads to a 21.1 % increase compared to the dark fermentation of non-pretreated algal biomass. In the same way, VFA and bio-hydrogen production increased by 8.7 % and 56.0 %, respectively, as a consequence of the pretreatment.
A possible way to improve RS, VFA and biohydrogen yields achieved in this study could be a chemical agent incorporation during the sonication process.
Practical applications
In this work, the valorisation of the biomass of the invasive seaweed R. okamurae for the production of RS and VFAs has been studied. The extensive proliferation of this invasive species along the coasts of Andalusia is currently considered a mayor environmental and socio-economic problem. Therefore, its use will allow the recovery of this algal biomass, while minimising the negative effects associated with its extensive colonisation. The processes used to convert this invasive algae species into biocommodities are applicable to any type of coastal algal deposit. It should be noted that the algal biomass has a low lignin content, so a mild pre-treatment such as that used in this study would be applicable to any other type of coastal macroalgae deposit.
Furthermore, as mentioned above, the RS and VFAs obtained from the proposed processes (enzymatic hydrolysis and dark fermentation) can be used in several biorefinery processes to produce high value-added end products. For example, VFAs can be used as a fermentation substrate for the production of medium chain fatty acids (MCFA), such as caproic acid, with multiple industrial applications in sectors as diverse as cosmetics, food, agronomy and energy. One of the main applications of the products derived from this processing of algal biomass are polyhydroxyalkanoates. These are bio-based, biodegradable bioplastic precursors that can be obtained by fermentation using both sugars and volatile fatty acids as carbon sources. The huge global environmental problem currently posed by plastic pollution makes this application of the products obtained particularly relevant. Furthermore, the biohydrogen produced during the dark fermentation process represents a promising biofuel as an alternative to fossil fuels.
Future research prospects
As mentioned above, the products derived from algae biomass are biocommodities for biorefineries and can be used in a variety of fermentation processes, including the production of MCFAs and PHAs. This requires further studies to optimise the pre-treatment and the fermentation processes themselves. With regard to the pre-treatment used, it is postulated that the use of a chemical agent during the ultrasound process may be a potential avenue for improvement. Recent studies on this invasive alga have demonstrated the potential for the production of bioactive compounds. It would therefore be beneficial to extract these compounds or alginate, which is a major component of brown algae, prior to the proposed bioprocessing for the production of sugars and volatile fatty acids. This would result in a more efficient use of the algal biomass, thereby increasing the economic viability of the proposed treatment.
References
Aboudi K, Fernández-Güelfo LA, Álvarez-Gallego CJ, Ahmedc B, Tyagic VK, Romero-García LI (2021) Chapter 19 - Polyhydroxyalkanoate production from algal biomass. In: Tyagi V, Aboudi K (eds) Clean Energy and Resources Recovery. Elsevier, Amsterdam, pp 447–464
Agabo-García C, Romero-García LI, Álvarez-Gallego CJ, Blandino A (2023) Valorisation of the invasive alga Rugulopteryx okamurae through the production of monomeric sugars. Appl Microbiol Biotechnol 107:1971–1982
Altamirano M, De la Rosa J, Muñoz A-R, Ros M (2019) Workshop de especies invasoras marinas: construyendo una propuesta para Andalucía Conclusiones de la mesa redonda. Algas 55:41–43
Arias A, Feijoo G, Moreira MT (2023) Macroalgae biorefineries as a sustainable resource in the extraction of value-added compounds. Algal Res 69:102954
Azizi N, Najafpour G, Younesi H (2017) Acid pretreatment and enzymatic saccharification of brown seaweed for polyhydroxybutyrate (PHB) production using Cupriavidus necator. Int J Biol Macromol 101:1029–1040
Barcellos L, Pham CK, Menezes G, Bettencourt R, Rocha N, Carvalho M, Felgueiras HP (2023) A concise review on the potential applications of Rugulopteryx okamurae macroalgae. Mar Drugs 21:40
Bridgewater LL (ed) (2017) Standard methods for the examination of water and wastewater, 23rd edn. American public health association, Washington, DC
Borines MG, de Leon RL, Cuello JL (2013) Bioethanol production from the macroalgae Sargassum spp. Bioresour Technol 138:22–29
Chemat F, Rombaut N, Sicaire A-G, Meullemiestre A, Fabiano-Tixier A-S, Abert-Vian M (2017) Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason Sonochem 34:540–560
Cui R, Zhu F (2021) Ultrasound modified polysaccharides: a review of structure, physicochemical properties, biological activities and food applications. Trends Food Sci Technol 107:491–508
de la Lama-Calvente D, Fernández-Rodríguez MJ, Llanos J, Mancilla-Leytón JM, Borja R (2021) Enhancing methane production from the invasive macroalga Rugulopteryx okamurae through anaerobic co-digestion with olive mill solid waste: process performance and kinetic analysis. J Appl Phycol 33:4113–4124
Dobrinčić A, Balbino S, Zorić Z, Pedisić S, Bursać Kovacević D, Garofulić IE, Dragović-Uzelac V (2020) Advanced technologies for the extraction of marine brown algal polysaccharides. Mar Drugs 18:168
Espada-Bellido E, Ferreiro-González M, Carrera C, Palma M, Barroso CG, Barbero GF (2017) Optimization of the ultrasound-assisted extraction of anthocyanins and total phenolic compounds in mulberry (Morus nigra) pulp. Food Chem 219:23–32
Estévez RM, Palacios M, Cervera JL, González Duarte MM (2022) Expansion of the invasive alga Rugulopteryx okamurae (Dictyotaceae, Ochrophyta) in the Mediterranean Sea: first evidence as epiphyte of the cold-water coral Dendrophyllia ramea (Cnidaria: Scleractinia). BioInvas Rec 11:925-936
Farghali M, Mohamed IMA, Osman AI, Rooney DW (2023) Seaweed for climate mitigation, wastewater treatment, bioenergy, bioplastic, biochar, food, pharmaceuticals, and cosmetics: a review. Environ Chem Lett 21:97–152
Fernandes H, Martins N, Vieira L, Salgado JM, Castro C, Oliva-Teles A, Belo I, Peres H (2022) Pre-treatment of Ulva rigida improves its nutritional value for European seabass (Dicentrarchus labrax) juveniles. Algal Res 66:102803
Fernández-Medina P, Álvarez-Gallego CJ, Caro I (2022) Yield evaluation of enzyme hydrolysis and dark fermentation of the brown seaweed Rugulopteryx okamurae hydrothermally pretreated by microwave irradiation. J Environ Chem Eng 10:108817
Ferreira-Anta T, Flórez-Fernández N, Torres MD, Mazón J, Dominguez H (2023) Microwave-assisted hydrothermal processing of Rugulopteryx okamurae. Mar Drugs 21:319
Flint EB, Suslick KS (1991) The temperature of cavitation. Science 253:1397–1399
Florido M, Megina C, García J (2023) Coexistiendo con una invasora en el estrecho de Gibraltar: la integración de Rugulopteryx okamurae en la fauna y flora residente. Almoraima Revista de Estudios Campogibraltareños 58:233–248
García-Gómez JC, Sempere-Valverde J, González AR, Martínez-Chacón M, Olaya-Ponzone L, Sánchez-Moyano E, Ostalé-Valriberas E, Megina C (2020) From exotic to invasive in record time: the extreme impact of Rugulopteryx okamurae (Dictyotales, Ochrophyta) in the strait of Gibraltar. Sci Total Environ 704:135408
Garcia-Vaquero M, Rajauria G, Tiwari B, Sweeney T, O’Doherty J (2018) Extraction and yield optimisation of fucose, glucans and associated antioxidant activities from Laminaria digitata by applying response surface methodology to high intensity ultrasound-assisted extraction. Mar Drugs 16:257
Gonçalves C, Rodriguez-Jasso RM, Gomes N, Teixeira JA, Belo I (2010) Adaptation of dinitrosalicylic acid method to microtiter plates. Anal Meth 2:2046
Karray R, Hamza M, Sayadi S (2015) Evaluation of ultrasonic, acid, thermo-alkaline and enzymatic pre-treatments on anaerobic digestion of Ulva rigida for biogas production. Bioresour Technol 187:205–213
Kim B, Jeong J, Kim J, Yoon HH, Thinh Nguyen PK, Kim J (2022) Mathematical modeling of dark fermentation of macroalgae for hydrogen and volatile fatty acids production. Bioresour Technol 354
Kumar G, Ponnusamy VK, Bhosale RR, Shobana S, Yoon J-J, Bhatia SK, Banu RJ, Kim S-H (2019) A review on the conversion of volatile fatty acids to polyhydroxyalkanoates using dark fermentative effluents from hydrogen production. Bioresour Technol 287:121427
Lara A, Rodríguez-Jasso RM, Loredo-Treviño A, Aguilar CN, Meyer AS, Ruiz HA (2020) Enzymes in the third generation biorefinery for macroalgae biomass. In: Singh SP, Pandey A, Singhania RR, Larroche C, Li Z (eds) Biomass, Biofuels, Biochemicals. Advances in enzyme catalysis and technologies. Elsevier, Amsterdam, pp 363–396
Liu H, Wang G (2014) Fermentative hydrogen production from macro-algae Laminaria japonica using anaerobic mixed bacteria. Int J Hydrogen Energy 39:9012–9017
Palma M, Barbeiro GF, Piñeiro Z, Liazid A, Barroso CG, Prado J, Meireles MAA (2014) Extraction of natural products principles and fundamental aspects. In: Rosragno MA, Prado JM (eds) Natural Product Extraction. In: Natural Product Extraction: Principles and Applications. RSC Publishing, Cambridge, pp 58–88
Naseem S, Rizwan M, Durrani AI, Munawar A, Gillani SR (2024) Innovations in cell lysis strategies and efficient protein extraction from blue food (Seaweed). Sust Chem Pharm 39:101586
Olatunji KO, Madyira DM, Amos JO (2024) Sustainable enhancement of biogas and methane yield of macroalgae biomass using different pretreatment techniques: A mini-review. Energy Environ 35:1050–1088
Pardilhó S, Cotas J, Pacheco D, Gonçalves AMM, Bahcevandziev K, Pereira L, Figueirinha A, Dias JM (2023) Valorisation of marine macroalgae waste using a cascade biorefinery approach: exploratory study. J Cleaner Product 385:135672
Park M-R, Jeong G-T (2021) Production of reducing sugar in Gracilaria verrucosa using physio-chemical pretreatment and subsequent enzymatic hydrolysis. Algal Res 60:102531
Passos F, Carretero J, Ferrer I (2015) Comparing pretreatment methods for improving microalgae anaerobic digestion: Thermal, hydrothermal, microwave and ultrasound. Chem Eng J 279:667–672
Pereira L (2021) Macroalgae. Encyclopedia 1:177–188
Rayo-Mendez LM, Koshima CC, Pessoa Filho PA, Tadini CC (2021) Recovery of non-starch polysaccharides from ripe banana (Musa cavendishii). J Food Eng 292:110256
Ribeiro RSA, Pohlmann BC, Calado V, Bojorge N, Pereira N Jr (2019) Production of nanocellulose by enzymatic hydrolysis: Trends and challenges. Eng Life Sci 19:279–291
Roca M, Dunbar MB, Román A, Caballero I, Zoffoli ML, Gernez P, Navarro G (2022) Monitoring the marine invasive alien species Rugulopteryx okamurae using unmanned aerial vehicles and satellites. Front Mar Sci 9:1004012
Romero-Vargas A, Fdez-Güelfo LA, Blandino A, Díaz MJ, Díaz AB (2023a) Rugulopteryx okamurae: effect of hydrothermal acid pretreatment on the saccharification process. Bioresour Technol 388:129721
Romero-Vargas A, Muñoz I, Marzo C, Díaz AB, Romero-García LI, Blandino A (2023b) Ultrasound pretreatment to enhance the enzymatic hydrolysis of Dictyota dichotoma for sugars production. Algal Res 71:103083
Romero-Vargas A, Fdez-Güelfo LA, Blandino A, Romero-García LI, Díaz AB (2023c) Rugulopteryx okamurae: assessment of its potential as a source of monosaccharides for obtaining bio-products. Chem Eng J 468:143578
Saleem R, Osama M, Khadim S, Farooqi U, Advani RS, Unar K, Unar AA, Baloch N, Siyal FJ, Shaikh B, Zafar J, Fatima, (2024) A Systematic review to unveil therapeutic potential of some common green seaweeds. J Survey Fish Sci 11:92–97
Saratale RG, Cho S-K, Saratale GD, Kumar M, Bharagava RN, Varjani S, Kadam AA, Ghodake GS, Palem RR, Mulla SI, Kim D-S, Shin H-S (2021) An overview of recent advancements in microbial polyhydroxyalkanoates (PHA) production from dark fermentation acidogenic effluents: A path to an integrated bio-refinery. Polymers 13:4297
Saravana PS, Alaydi H, Cabral EM, Poojary MM, Karuppusamy S, Tiwari BK (2024) Ultrasound, microwave and enzyme-assisted multiproduct biorefinery of Ascophyllum nodosum. Food Chem 433:137259
Saravanan K, Duraisamy S, Ramasamy G, Kumarasamy A, Blakrishnan S (2018) Evaluation of the saccharification and fermentation process of two different seaweeds for an ecofriendly bioethanol production. Biocatal Ag Biotech 14:444–449
Sekoai PT, Ghimire A, Ezeokoli OT, Rao S, Ngan WY, Habimana O, Yao Y, Yang P, Yiu Fung AH, Yoro KO, Daramola MO, Hung C-H (2021) Valorization of volatile fatty acids from the dark fermentation waste streams - a promising pathway for a biorefinery concept. Renew Sustain Energy Rev 143:110971
Shabarish S, Tamilarasan K, Rajesh Banu J, Godvin Sharmila V (2023) Biohydrogen production from macroalgae via sonic biosurfactant disintegration: an energy efficient approach. Resour Environ Sustain 11:100093
Shankaran S, Karuppiah T, Jeyakumar RB (2022) Chemo-sonic pretreatment approach on marine macroalgae for energy efficient biohydrogen production. Sustainability 14:12849
Sharma P, Melkania U (2017) Impact of furan derivatives and phenolic compounds on hydrogen production from organic fraction of municipal solid waste using co-culture of Enterobacter aerogenes and E. coli. Bioresour Technol 239:49–56
Sidana A, Yadav SK (2022) Recent developments in lignocellulosic biomass pretreatment with a focus on eco-friendly, non-conventional methods. J Cleaner Product 335:130286
Snehya AV, Sundaramahalingam MA, Rajeshbanu J, Anandan S, Sivashanmugam P (2021) Studies on evaluation of surfactant coupled sonication pretreatment on Ulva fasciata (marine macroalgae) for enhanced biohydrogen production. Ultrason Sonochem 81:105853
Snehya AV, Sundaramahalingam MA, Rajeshbanu J, Sivashanmugam P (2022) Studies on evaluation of pretreatment efficiency of surfactant mediated ultrasonic pretreatment of Sargassum tennerimum (marine macroalgae) for achieving profitable biohydrogen production. Int J Hydrogen Energy 47:17184–17193
Sun C, Liao Q, Xia A, Fu Q, Huang Y, Zhu X, Zhu X, Wang Z (2020) Degradation and transformation of furfural derivatives from hydrothermal pre-treated algae and lignocellulosic biomass during hydrogen fermentation. Renew Sustain Energy Rev 131:109983
Thompson TM, Young BR, Baroutian S (2019) Advances in the pretreatment of brown macroalgae for biogas production. Fuel Process Technol 195:106151
Yi Y, Xu W, Wang H-X, Huang F, Wang L-M (2020) Natural polysaccharides experience physiochemical and functional changes during preparation: a review. Carbohydr Polym 234:115896
Yin Y, Hu J, Wang J (2019) Fermentative hydrogen production from macroalgae Laminaria japonica pretreated by microwave irradiation. Int J Hydrogen Energy 44:10398–10406
Youssouf L, Lallemand L, Giraud P, Soulé F, Bhaw-Luximon A, Meilhac O, D’Hellencourt CL, Jhurry D, Couprie J (2017) Ultrasound-assisted extraction and structural characterization by NMR of alginates and carrageenans from seaweeds. Carbohydr Polym 166:55–63
Zhang D, Jiang H, Chang J, Sun J, Tu W, Wang H (2019) Effect of thermal hydrolysis pretreatment on volatile fatty acids production in sludge acidification and subsequent polyhydroxyalkanoates production. Bioresour Technol 279:92–100
Funding
Funding for open access publishing: Universidad de Cádiz/CBUA Grant TED2021-130891R-I00 funded by MICIU/AEI/10.13039/501100011033 and by European Union NextGenerationEU/PRTR and the R+D+I project PID2019-104525RB-I00 funded by MCIN/AEI/10.13039/501100011033/. The authors also acknowledge the MCIN for the scholarship PRE2020-092698.
Author information
Authors and Affiliations
Contributions
Conceptualization, L.L., B.A. and R-G.LI.; methodology, L.L., B.A. and R-G.LI.; software, L.L.; validation, B.A. and R-G.LI.; formal analysis, B.A. and R-G.LI.; investigation, L.L., F-Z.E., R-V.A.; data curation, L.L., F-Z.E., R-V.A., B.A. and R-G.LI.; writing—original draft preparation, L.L.; writing—review and editing, B.A. and R-G.LI.; visualization and supervision, B.A. and R-G.LI.; project administration, B.A.; funding acquisition, B.A. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests
Data availability
The data presented in this study are available on request from the corresponding author.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
León-Marcos, L., Fuente-Zapico, E., Romero-Vargas, A. et al. Ultrasound pretreatment of third-generation biomass (invasive macroalga Rugulopteryx okamurae) to obtain platform biocommodities. J Appl Phycol (2024). https://doi.org/10.1007/s10811-024-03316-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10811-024-03316-9