Abstract
The multibillion-dollar carbonated beverage industry is currently facing questions from health-conscious consumers over negative health effects of such beverages. Decreasing consumption trends have forced companies to look for healthier choices for their products. C-phycocyanin CPC, a bright blue cyanobacterial pigment with anti-oxidant and other health benefits has been proposed as a candidate in edible drinks. We found that CPC is stable in a wide pH and temperature regime. Reaction kinetics for 12 weeks at 4 °C in non-alcoholic carbonated beverages (B1-B4) showed that B3 (sweetened, ~30 % degradation) best preserved CPC integrity while B1 (non-sweetened, ~87 % degradation) was ineffective. Other beverages (sweetened) could preserve ~ 49 % CPC integrity. Behnajady-Modirshahla-Ghanbary and first order kinetic models explained CPC degradation with and without preservative (sucrose), respectively. The ’consume-by’ times suggest possible refrigeration from ~ 13 hours to 27 days for various CPC-containing beverages. Results suggest CPC could be filter-sterilized and added to non-alcoholic beverages before being packaged in cans or tetra packs to avoid light exposure.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-alcoholic carbonated beverages (NACBs) are a major player in the food industry. Although their consumption has recently shown a decreasing trend owing to health concerns, they remain a major component of the daily diet. Recent data show an average per capita consumption of non-alcoholic carbonated beverages of 146 – 150 L per year in the USA (IBISWorld 2023; statista 2022). Other major consumers include Mexico, Canada, Brazil, Japan, and Russia (statista 2022). They are thus a target of experimental and innovative products. NACBs have carbon dioxide dissolved in them under pressure in addition to stabilizers. Sweetened versions generally have sugar, high fructose corn syrup or artificial, low-calorie sweeteners in addition to acidity regulators, preservatives, and coloring agents. Recently, companies are turning to healthier alternatives for color, to appeal to health-conscious consumers.
Phycobiliproteins are water soluble, fluorescent proteins found in cyanobacteria, red algae, and cryptophytes as part of their accessory light harvesting complex, the phycobilisome. In the photosynthetic process they capture light at wavelengths inaccessible to chlorophyll. C-phycocyanin (CPC) is one of the phycobiliproteins found in cyanobacteria and facilitates light energy transfer between phycoerythrin and allophycocyanin, the other two forms of phycobiliprotein (Ghosh et al. 2015; 2020). CPC is the most abundant of the phycobiliproteins and can make up to 20 % of the total proteins in cyanobacteria (dry weight basis) (Renaudin et al. 2021). On a dry weight basis, phycocyanin content can range from ~ 3.7 to 4.4 % (Leptolyngbya boryana CCALA 084) to ~ 5.5 to 17.5 % (Arthrospira platensis) (Patel et al. 2005; Basheva et al. 2018; Khandual et al. 2021). Its absorption maximum is in the range of 615 - 620 nm and it fluoresces around 649 – 651 nm. The fluorescence emanates from linear tetrapyrrole molecules called phycocyanobilins attached to specific cysteine residues in the amino acid backbone (Ghosh et al. 2020). Apart from its role in light harvesting, the natural fluorescence of CPC has been utilized for many different applications such as fluorescence activated cell sorting, anti-oxidant activity, small ion and DNA probe and as biochemical tracers in immunoassays (Oi et al. 1982; Telford et al. 2001; Thangam et al. 2013; Bhayani et al. 2016; Ghosh et al. 2020). Recently, CPC has also attracted attention for its applicability in the food sector. Its brilliant blue color and stability under wide pH and temperature ranges has contributed to its increasing commercial acceptance. It is also the only natural blue algal pigment approved in the USA and Europe for use in food products (DIC Corporation 2022). Coupled with its anti-oxidant, anti-nephrolithe, anti-hyperglycemic, and other beneficial activities (Paliwal et al. 2015; Ghosh et al. 2016; Liu et al. 2022; Fernandes et al. 2023), CPC is expected to appeal to consumers. However, since its application in the food industry is dependent on CPC meeting requirements regarding stability and degradation, several reports have focused on the degradation of CPC in vitro and ways to improve upon it using preservatives and other stabilizing agents and procedures (Braga et al. 2016; Kannaujiya and Sinha 2016; Hadiyanto et al. 2018; 2019; Böcker et al. 2019; Faieta et al. 2020; 2022; Zhong et al. 2024; Zhou et al. 2024). If we focus on relevant studies utilizing actual food articles off the supermarket shelves, the number diminishes rapidly. One recent article studied the color stability of CPC in isotonic and tonic beverages for a 11-day period, with no significant degradation (García et al. 2021). However; there is an urgent need for studies detailing the kinetics of long-term storage of CPC mixed with such beverages. This work assesses the stability of CPC under different pH, temperature, and light exposure regimes, the utility of CPC as a food colorant in NACBs, and the degradation kinetics for a longer duration of 12 weeks to gain a more comprehensive picture on the applicability of CPC in currently marketed beverages.
Materials and Methods
Microorganism cultivation
Arthrospira sp. was taken from the culture collection at CSIR - Central Salt & Marine Chemicals Research Institute and grown in Zarrouk’s medium (Patel et al. 2005) with a 14:10 light:dark cycle and a temperature of 25 ± 2 °C. The cultures were agitated manually twice a day to prevent adherence to flask walls.
CPC extraction and purification
The extraction procedure was adopted from a previously reported protocol (Mishra et al. 2008). Briefly, the harvested biomass was freeze thawed twice in 0.1 M phosphate buffer (pH 7.0) followed by 25 % and then 50 % ammonium sulphate precipitation. The resultant pellet was subjected to acetate buffer treatment (0.1 M, pH 4.5) for 10 min before being recovered using 50 % ammonium sulphate precipitation and dialyzed against 0.1 M phosphate buffer (pH 7.0) overnight with four buffer changes (12-14 kDa cutoff dialysis membrane, holding capacity ~ 3.63 mL cm-1, HiMedia Laboratories, India).
CPC estimation and purity ratio
The spectra of the samples were recorded bi-weekly using a UV – visible spectrophotometer (Cary 50 Bio, Varian Inc., USA). Estimation of the CPC content and its purity ratio was determined according to the equations of Bennett and Bogorad (1973):
where A618, A652, and A280 signify absorption at 618, 652, and 280 nm, respectively. A protein dilution of ~100 - 150 µg mL-1 was prepared either in buffers or the respective beverages for further studies.
Stability of CPC in different pH, temperatures, and light
Purified CPC was stored for 30 min in different pH (2 - 11) and for 48 h under a light intensity of 43 µmol photons m-2 s-1 (~ 3000 lux with cool white fluorescent lights) at a temperature of 4 ± 2 °C. It was also stored for 2 weeks at different temperatures (-60, 4 and 10 °C) to assess the effect of storage. Additionally, CPC was exposed to different temperatures from 4 – 100 °C for 2 h for investigating the effects of short-term storage (Ghosh and Mishra 2020).
Kinetic studies
The stability of the purified CPC was studied by storing the CPC supplemented beverages (~ 100 – 150 µg mL-1 CPC concentration) at 4 ± 2 °C for 12 weeks. Phosphate (0.1 M, pH 7.0) and acetate (0.1 M, pH 4.5) buffers were used as controls. Spectra of the samples were recorded at regular intervals and the CPC concentration was determined as per Eq. 1. The data obtained were fitted using different kinetic models as discussed further. The zero, first and second order kinetics are given by Eqs 3–5:
where [Co] and [C] denote the initial and final concentrations of CPC while k is the rate constant and t denotes the time of reaction. The rate constant k was determined from the appropriate plots of CPC concentration vs. time.
The Behnajady-Modirshahla-Ghanbary (BMG) model is given by the following equation (Behnajady et al. 2007):
where [C] and [C0] are final and initial concentrations of CPC and t is time. Since this model was first proposed for explaining the kinetics of dye degradation through Fenton’s oxidation, the constants m and b have been defined for that process. Higher 1/m value characterizes a rapid initial decay of the protein while the term 1/b denotes the theoretical maximum of CPC removed from the solution through degradation as the time approaches infinity. Both m (intercept) and b (slope) can be calculated from a plot of \(\tt \frac{{\text{t}}}{(1-\frac{\left[C\right]}{\left[C_{o}\right]})}\) vs. t (Behnajady et al. 2007).
The ‘consume-by’ time was taken as the time by which the beverages retained 90 % of the initial CPC content after being opened. It was calculated for the first order and BMG models by Eqs. 7 and 8 respectively:
where k is the first order rate constant and m and b are the BMG model constants.
Statistical analysis
All results are presented as mean ± SD (n = 3). Fisher LSD test was used for evaluating the statistical significance using InfoStat Student (v2020) (Di Rienzo et al. 2011); the difference was considered significant at p < 0.01.
Results
CPC purification
The purified CPC showed an absorbance peak at 618 nm (Fig. 1) and had a purity ratio of 1.21. There were no shoulder peaks at ~ 560 and 652 nm, which showed the absence of phycoerythrin and allophycocyanin, the other 2 members of the phycobiliprotein family and most common contaminants. The CPC was quantified according to Eq. 1 and diluted to a final concentration (~ 100 - 150 µg mL-1) using buffers or beverages according to requirements.
Stability of CPC in different pH, temperatures, and light
Stability of CPC in different pH
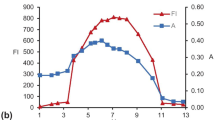
The stability of a potential food additive needs to be investigated over a broad pH range to assess its suitability for usage in the food industry. CPC was stored for 30 min in buffers of different pH (2 – 11) (Fig. 2). It was stable within a pH range of 4 – 8 with an average concentration ranging from ~89.29 - 100 %, which could be an advantage given that the beverages are often acidic in nature. Extreme pH values, both acidic and basic, were detrimental to the protein’s stability, and adversely affected the optical characteristics of the bilin moieties as well. The protein precipitated at pH 2 while it started degrading when the pH was increased to 9 or above. Further increase caused a complete disappearance of the bilin absorbance peak, which signaled significant protein denaturation (Fig. S1, Electronic Supplementary Information, ESI).
Stability of CPC under light exposure
CPC is essentially a light harvesting protein and is usually purified and stored in the dark. The possibility of using CPC as a food colorant depends in part on its stability under light exposure. For this reason, the extracted protein was exposed to a light intensity of 43 µmol photons m-2 s-1 for 48 h (0.1 phosphate buffer, pH 7.0) to study its stability (Fig 3). CPC did not show any significant changes in its optical properties till 12 h of light exposure; it had a residual concentration of ~ 89.55 – 95.94 % between 1 – 12 h of exposure, but its stability deteriorated afterwards. The residual CPC after 24 and 48 h of exposure was 68.28 and 34.72 % of the initial, respectively. The spectral scans showed significant degradation after 12 hours (Fig. S2, ESI).
Stability of CPC in different temperatures
Food additives are frequently subjected to temperature variations during their processing as well as storage. Thus, we needed to investigate the efficacy of CPC after long- and short-term storage at different temperatures. For the long-term study, the extracted protein was stored at -60, 4 and 10 °C (0.1 M phosphate buffer, pH 7.0, Fig. S3a, S3b, and S3c respectively, ESI) in the dark for 2 weeks to assess its stability under extended storage. Fig. 4a shows the CPC remaining after the 2-week storage period in different samples indicating that -60 °C was best for CPC storage, which was expected since such low temperatures reduce the water activity and limit microbial degradation. Maximum degradation occurred within the first 6 h of storage and the extent gradually reduced. However, extended storage at 10 °C led to maximum degradation (~38 % decrease compared to initial concentration). In comparison, the samples stored at -60 and 4 °C showed ~24 % and ~25% decrease in the CPC concentration at the end of the experiment.
a) Remnant C-phycocyanin after 2-week storage at different temperatures (-60, 4, and 10 °C), and b) Remnant C-phycocyanin after a 2-h exposure to different temperatures (4 – 100 °C, 0.1 M phosphate buffer, pH 7.0) (arranged in decreasing order of significance, values with different letters are significantly different at p < 0.01)
To assess the thermal stability of CPC, it was also subjected to short-term storage at different temperatures (4 – 100 °C) for 2 h (Fig. 4b). CPC content decreased from 2.86 – 13.04 % in the stored samples as the temperature increased from 10 to 50 °C compared to the sample stored at 4 °C. However, a loss of the bilin peak at 618 nm was observed when the temperature was increased to 60 and 100 °C (Fig. S3d, ESI); the protein sample was completely discolored at 100 °C with the formation of aggregates at the bottom.
Kinetic studies
Kinetic studies on CPC degradation allow the modeling of their behavior in vitro and the design of process parameters for minimizing loss. CPC was stored in sweetened and non-sweetened carbonated beverages for 12 weeks at 4 ± 2 °C with buffers serving as the control and regular monitoring of the remnant concentration; the beverages' composition are detailed in Table 1 and the results are presented in Fig. 5; the kinetic parameters of the models are presented in Table 2. Since the protein was dissolved and stored in opened beverages, food spoilage from air-borne microorganisms also affects its utility. To present a more complete picture, we have also included calculations on the ‘consume-by’ date which considers the time taken by CPC to reduce to 90 % of its initial concentration (Table 2).
Buffers
In the absence of any preservatives, phosphate buffer was unable to stop the degradation of the protein during the storage period; the acetate buffered sample showed CPC degradation of ~35 % compared to ~90 % in the phosphate buffered one at the end of the experiment (Fig. 5). The degradation kinetics were found to follow BMG model in acetate buffer (Fig. S4a and S4b, ESI) while the phosphate buffered sample followed first order kinetics (Fig. S4c and S4d, ESI) (Table 2). Visually, the phosphate buffered sample was completely colorless at the end of the 12-week storage period, while the acetate buffered sample retained its color to some extent (Fig. 6).
Non sweetened carbonated beverage
The study was designed to assess the use of CPC in actual beverages; B1 did not contain any added sucrose (Table 1) which has earlier been reported for preserving the CPC integrity (Mishra et al. 2008; Chaiklahan et al. 2012). Predictably, the degradation in B1 followed first order kinetics (Table 2) with ~ 87 % loss of CPC integrity after 12 weeks and the degradation profile following a similar path as the phosphate buffered sample (Fig. 5). B1 was the least performing beverage in terms of CPC preservation (Fig. 5); the degree of denaturation is evident by the complete absence of the characteristic phycocyanobilin peak at 618 nm after 12 weeks (Fig. S5a, ESI) as well as the total loss of color (Fig. 6). Apart from the presence of CO2, B1 was not very different from phosphate buffered sample, and it expectedly followed the same kinetics (Fig. S5b, ESI). The ‘consume-by’ date after opening the beverage was calculated to be ~ 105 h, which translates to a little more than 4 days.
Sweetened carbonated beverages
The other 3 (B2 – B4) beverages chosen were sweetened (Table 1) i.e., they had added sucrose in them which could act as a preservative for CPC in vitro. Expectedly, all the beverages performed better than B1 (Fig. 5). The addition of sucrose as a preservative had a significant role in prolonging the life of CPC in vitro; since it was the major ingredient present, the models also followed a similar pattern. The degradation was explained using the BMG model (Table 2) with varying degrees of strictness (Fig. S6a – S6f, ESI).
B3 was found to be the best beverage for CPC preservation, with ~30 % degradation in CPC concentration after the 12-week storage. B2 and B4 showed a CPC degradation of ~48 % and ~49 % after 12 weeks, respectively. The CPC color was the healthiest in B3 (Fig. 6). The maximum degradation occurred in the first 2 weeks following the start of the experiment; thereafter, the samples showed minimal degradation until the end (Fig. 5). The ‘consume-by’ date told a similar story. It was found that B3 had a considerably longer consumption life after opening (~ 647 h or ~ 27 days) compared to B2 (~ 78 h or ~ 3 days) and B4 (~ 13.5 h). In order to put these values in a perspective for the reader, aseptically packed milk opened after purchase has a 'consume-by' period of 7 -10 days (Bell 2023).
Discussion
CPC stability studies in pH, temperature, and light exposure conditions
Phycobiliproteins are primarily accessory light harvesting pigments; in their physiological role, they are constantly replenished by the cellular machinery. However, as proteins they are sensitive to pH, temperature, and light exposure. We observed that CPC had an optimum pH, temperature, and light exposure beyond which its degradation was more pronounced and rapid. In their biological setting, the tertiary protein structure exposes the phycocyanobilins to light for picoseconds (Glazer 1989) and the protein molecules are constantly replaced by biosynthesis. However, after extraction, the protein stability in vitro is affected by a multitude of factors. Some of the studies have reported CPC instability in different pH regimes, especially when heating is involved (Ghosh and Mishra 2020; Adjali et al. 2021; Buecker et al. 2022). Munier et al (2014) have also reported the stability of phycoerythrin, another member of the phycobiliprotein family, in a broad pH range of 4 – 10 and for up to ~8 h under light exposure which mirrors our own observations on CPC. The use of additives such as alginate and maltodextrin for improving the tolerance of CPC to light and increase its shelf life has also been discussed (Adjali et al. 2021; Nowruzi et al. 2022). Moreover, light mediated CPC degradation has also been reported (Wu et al. 2016; Aoki et al. 2021). However, most studies suggest avoiding light exposure and extreme pH variations to preserve CPC integrity, since extreme pH cause aggregation and disruption of the tertiary structure while light exposure adversely affects the bilins’ pigmentation.
Similarly, degradation above 50 °C was expected since the source organism is mesophilic. Increasing thermal energy leads to opening of the protein tertiary structure which exposes the bilins to heat, thus leading to denaturation. Thermal denaturation of CPC has also been reported in several studies. Most of them report denaturation above temperatures of 60 – 70 °C; the rapidity increases in the presence of unfavorable pH or light intensity (Adjali et al. 2021). One study has reported phycoerythrin stability in temperatures up to 40 °C (Munier et al. 2014). Our group has earlier reported thermal instability of a related protein, C-phycoerythrin, at temperatures > 40 °C (Ghosh and Mishra 2020); looking at the results, resistance to such short term temperature fluctuations could actually be advantageous for CPC since it increases its practical applications. From our own observations, it is suggested that CPC, being stable in a wide pH range (4 – 8) and able to tolerate continuous light exposure for up to 12 h, can be a potential pigmentation candidate in acidic beverages. The extended stability at 4 °C was also very encouraging for us since such a temperature regime is practical and convenient to implement. Overall, the bottling plant could filter sterilize and refrigerate CPC; for maximum integrity, CPC should be added just before packaging and the drinks could be stored under refrigeration in sealed cans or tetra packs, as opposed to clear plastic or glass bottles.
CPC degradation kinetics in beverages
The degradation kinetics enable mathematical visualization of the CPC degradation process, and help to design processes which can enhance its longevity. Protein extraction and storage buffers normally have some preserving agent, like sodium azide, added to them. However, in case of CPC, acetate itself acts as a preservative; its preserving effect has been observed earlier (Mishra et al. 2008; Chaiklahan et al. 2012; Kannaujiya and Sinha 2016; Fernandes et al. 2023). This explains the greater stability of CPC in acetate compared to the phosphate buffer. Another interesting fact was that, according to a recent review, the first order kinetic model is common in many CPC degradation studies (Adjali et al. 2021). Non-sweetened beverages only have dissolved CO2 in them. The absence of any other preservative quickens the pace of degradation, as was seen in B1. These observations suggest that CPC may not be a very good candidate as an additive for non-sweetened drinks unless additional stabilization procedures are included. The ‘consume-by’ date of ~ 4 days reminded us that opened beverages with added CPC can be a target of airborne microorganisms and other degrading factors; the beverage should be consumed within this period.
If we look at the behavior of CPC in sweetened beverages (B2 – B4), the role of sucrose as a preservative becomes quite clear. Further, the BMG model provides an excellent approximation, with varying strictness, for CPC degradation whenever a preservative (sucrose or acetate) is involved. It also suggests that CPC could be successfully used in sweetened carbonated beverages as a natural pigment. However, a longer ‘consume-by’ date in B3 suggests that the opened beverage can be refrigerated longer compared to those with shorter times (for example, in case of B2 or B4).
Studies describing the use of CPC as a coloring agent in beverages are very few. To the best of our knowledge, only one report has investigated the coloring ability of CPC in tonic and isotonic beverages and has concluded that the required CPC concentration for appropriate coloring ranges from ~16 – 112 mg L-1 (García et al. 2021), similar to our own observations. Furthermore, as a natural anti-oxidant, anti-nephrolithe, anti-hyperglycemic, anti-inflammatory, and an anti-cancer agent (Paliwal et al. 2015; Ghosh et al. 2016; Liu et al. 2022; Fernandes et al. 2023; Ziyaei et al. 2023), CPC will appeal to the health-conscious. The issues on sterilization and light and temperature sensitivities could be addressed within the framework of existing operations, which would make this switchover minimally intrusive for the companies.
Conclusion
In conclusion, this work investigated the applicability of CPC to be used as a coloring agent in sweetened and non-sweetened NACBs. Under proper packaging, sterilization, and storage, CPC could be a viable natural alternative. The degradation kinetics showed that the added preservatives in the beverages can play a role in stabilizing CPC. The data also showed that in the presence of preservatives such as acetate or sugar, CPC degradation tends to follow the BMG model while in their absence, it degrades according to first order kinetics. The ‘consume-by’ date had a range of ~ 0.5 – 27 days, which could possibly be managed with pack sizes. Considering the flak being faced by beverage companies worldwide, inclusion of CPC is going to be seen as a positive step forward for the entire food industry due to its health benefits, visual appeal, and wide applicability.
Data availability
All data supporting the findings of this study are available within the paper and its Supplementary Information.
References
Adjali A, Clarot I, Chen Z, Marchioni E, Boudier A (2021) Physicochemical degradation of phycocyanin and means to improve its stability: a short review. J Pharm Anal 12:406–414
Aoki J, Sasaki D, Asayama M (2021) Development of a method for phycocyanin recovery from filamentous cyanobacteria and evaluation of its stability and antioxidant capacity. BMC Biotechnol 21:40
Basheva D, Moten D, Stoyanov P, Belkinova D, Mladenov R, Teneva I (2018) Content of phycoerythrin, phycocyanin, allophycocyanin and phycoerythrocyanin in some cyanobacterial strains: Applications. Eng Life Sci 18:861–866
Behnajady MA, Modirshahla N, Ghanbary F (2007) A kinetic model for the decolorization of C.I. Acid Yellow 23 by Fenton process. J Hazard Mater 148:98–102
Bell C (2023) Here's how long milk really lasts-and how to make it last longer. Reader's digest. https://www.rd.com/article/how-long-milk-lasts/. Accessed 12 Mar 2024
Bennett A, Bogorad L (1973) Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol 58:419–435
Bhayani K, Mitra M, Ghosh T, Mishra S (2016) C-Phycocyanin as a potential biosensor for heavy metals like Hg2+ in aquatic systems. RSC Adv 6:111599–111605
Böcker L, Ortmann S, Surber J, Leeb E, Reineke K, Mathys A (2019) Biphasic short time heat degradation of the blue microalgae protein phycocyanin from Arthrospira platensis. Innov Food Sci Emerg Technol 52:116–121
Braga ARC, Figueira FDS, Silveira JTD, Morais MGD, Costa JAV, Kalil SJ (2016) Improvement of thermal stability of C-phycocyanin by nanofiber and preservative agents. J Food Process Preserv 40:1264–1269
Buecker S, Grossmann L, Loeffler M, Leeb E, Weiss J (2022) Thermal and acidic denaturation of phycocyanin from Arthrospira platensis: Effects of complexation with λ-carrageenan on blue color stability. Food Chem 380:132157
Chaiklahan R, Chirasuwan N, Bunnag B (2012) Stability of phycocyanin extracted from Spirulina sp.: Influence of temperature, pH and preservatives. Process Biochem 47:659–664
Di Rienzo J, Casanoves F, Balzarini M, Gonalez L, Tablada M, Robledo CW (2011) InfoStat ver. 24-03-2011, Universidad Nacional de Córdoba, Córdoba, Argentina. http://www.infostat.com.ar/. Accessed 31 Oct 2023
DIC Corporation (2022) Natural Blue Colorants. https://www.dic-global.com/en/products/natural_colorants/. Accessed 20 Nov 2023
Faieta M, Neri L, Sacchetti G, Di Michele A, Pittia P (2020) Role of saccharides on thermal stability of phycocyanin in aqueous solutions. Food Res Int 132:109093
Faieta M, Toong C, Corradini MG, Ludescher RD, Pittia P (2022) Degradation kinetics of C-phycocyanin under isothermal and dynamic thermal treatments. Food Chem 382:132266
Fernandes R, Campos J, Serra M, Fidalgo J, Almeida H, Casas A, Toubarro D, Barros AIRNA (2023) Exploring the benefits of phycocyanin: From Spirulina cultivation to its widespread applications. Pharmaceuticals 16:592
García AB, Longo E, Bermejo R (2021) The application of a phycocyanin extract obtained from Arthrospira platensis as a blue natural colorant in beverages. J Appl Phycol 33:3059–3070
Ghosh T, Mishra S (2020) Studies on extraction and stability of C-phycoerythrin from a marine cyanobacterium. Front Sustain Food Syst 4:102
Ghosh T, Paliwal C, Maurya R, Mishra S (2015) Microalgal rainbow colours for nutraceutical and pharmaceutical applications. In: Bahadur B, VenkatRajam M, Sahijram L, Krishnamurthy KV (eds) Plant biology and biotechnology: plant diversity, organization, function and improvement. Springer India, New Delhi, pp 777–791
Ghosh T, Bhayani K, Paliwal C, Maurya R, Chokshi K, Pancha I, Mishra S (2016) Cyanobacterial pigments as natural anti-hyperglycemic agents: an in vitro study. Front Mar Sci 3:146
Ghosh T, Mondal A, Vyas A, Mishra S (2020) A ‘one–tube’ synthesis of a selective fluorescence ‘turn off/on’ DNA probe based on a C-phycocyanin-graphene oxide (CPC-GO) bio composite. Int J Biol Macromol 163:977–984
Glazer AN (1989) Light guides: Directional energy transfer in a photosynthetic antenna. J Biol Chem 264:1–4
Hadiyanto H, Christwardana M, Sutanto H, Suzery M, Amelia D, Aritonang RF (2018) Kinetic study on the effects of sugar addition on the thermal degradation of phycocyanin from Spirulina sp. Food Biosci 22:85–90
Hadiyanto H, Christwardana M, Suzery M, Sutanto H, Nilamsari AM, Yunanda A (2019) Effects of carrageenan and chitosan as coating materials on the thermal degradation of microencapsulated phycocyanin from Spirulina sp. Int J Food Eng 15:20180290
IBISWorld (2023) Per capita soft drink consumption. https://www.ibisworld.com/us/bed/per-capita-soft-drink-consumption/1786/. Accessed 20 Mar 2024
Kannaujiya VK, Sinha RP (2016) Thermokinetic stability of phycocyanin and phycoerythrin in food-grade preservatives. J Appl Phycol 28:1063–1070
Khandual S, Sanchez EOL, Andrews HE, de la Rosa JDP (2021) Phycocyanin content and nutritional profile of Arthrospira platensis from Mexico: efficient extraction process and stability evaluation of phycocyanin. BMC Chem 15:24
Kraseasintra O, Tragoolpua Y, Pandith H, Khonkarn R, Pathom-aree W, Pekkoh J, Pumas C (2022) Application of phycocyanin from Arthrospira (Spirulina) platensis as a hair dye. Front Mar Sci 9:1024988
Liu R, Qin S, Li W (2022) Phycocyanin: Anti-inflammatory effect and mechanism. Biomed Pharmacother 153:113362
Mishra SK, Shrivastav A, Mishra S (2008) Effect of preservatives for food grade C-PC from Spirulina platensis. Process Biochem 43:339–345
Munier M, Jubeau S, Wijaya A, Morancais M, Dumay J, Marchal L, Jaouen P, Fleurence J (2014) Physicochemical factors affecting the stability of two pigments: R-phycoerythrin of Grateloupia turuturu and B-phycoerythrin of Porphyridium cruentum. Food Chem 150:400–407
Nowruzi B, Konur O, Anvar SAA (2022) The stability of the phycobiliproteins in the adverse environmental conditions relevant to the food storage. Food Bioproc Technol 15:2646–2663
Oi VT, Glazer AN, Stryer L (1982) Fluorescent phycobiliprotein conjugates for analyses of cells and molecules. J Cell Biol 93:981–986
Paliwal C, Ghosh T, Bhayani K, Maurya R, Mishra S (2015) Antioxidant, anti-nephrolithe activities and in vitro digestibility studies of three different cyanobacterial pigment extracts. Mar Drugs 13:5384–5401
Patel A, Mishra S, Pawar R, Ghosh PK (2005) Purification and characterization of C-phycocyanin from cyanobacterial species of marine and freshwater habitat. Protein Expr Purif 40:248–255
Renaudin M, Darnajoux R, Bellenger J-P (2021) Quantification of moss-associated cyanobacteria using phycocyanin pigment extraction. Front Microbiol 11:611792
Statista (2022) Per capita consumption of carbonated soft drinks in 2019 in the ten most populated countries worldwide. statista https://www.statista.com/statistics/505794/cds-per-capita-consumption-in-worlds-top-ten-population-countries/. Accessed 20 Mar 2024
Tahmassebi JF, BaniHani A (2020) Impact of soft drinks to health and economy: a critical review. Eur Arch Paediatr Dent 21:109–117
Telford WG, Moss MW, Morseman JP, Allnutt FCT (2001) Cryptomonad algal phycobiliproteins as fluorochromes for extracellular and intracellular antigen detection by flow cytometry. Cytometry 44:16–23
Thangam R, Suresh V, AsenathPrincy W, Rajkumar M, SenthilKumar N, Gunasekaran P, Rengasamy R, Anbazhagan C, Kaveri K, Kannan S (2013) C-Phycocyanin from Oscillatoria tenuis exhibited an antioxidant and in vitro antiproliferative activity through induction of apoptosis and G0/G1 cell cycle arrest. Food Chem 140:262–272
Wu H-L, Wang G-H, Xiang W-Z, Li T, He H (2016) Stability and antioxidant activity of food-grade phycocyanin isolated from Spirulina platensis. Int J Food Prop 19:2349–2362
Zhong Y, Sun S, Dai T, Zhang H, Wu J, Gong ES (2024) Phycocyanin-chitosan complex stabilized emulsion: preparation, characteristics, digestibility, and stability. Int J Biol Macromol 260:129253
Zhou Y, Huang Z, Liu Y, Li B, Wen Z, Cao L (2024) Stability and bioactivities evaluation of analytical grade C-phycocyanin during the storage of Spirulina platensis powder. J Food Sci 89:1442–1453
Ziyaei K, Abdi F, Mokhtari M, Daneshmehr MA, Ataie Z (2023) Phycocyanin as a nature-inspired antidiabetic agent: a systematic review. Phytomedicine 119:154964
Acknowledgements
TG would like to acknowledge the help and support from his colleagues at CSIR-Central Salt & Marine Chemicals Research Institute, Bhavnagar, India and the Institute for Water and Wastewater Technology, Durban, South Africa for their help and support during the conduct of this study.
Funding
Open access funding provided by Durban University of Technology. Science and Engineering Research Board (SERB), India (SERB grant no. PDF/2021/000144), The National Research Foundation - South African Research Chairs Initiative (NRF-SARChI grant no. 84166), South Africa, and the Durban University of Technology, South Africa. The funders had no role in study design, data collection and interpretation, manuscript writing, or the decision to publish the study.
Author information
Authors and Affiliations
Contributions
Conceptualization: Tonmoy Ghosh, Sandhya Mishra; Methodology, Investigation, and Formal Analysis: Tonmoy Ghosh, Ismail Rawat; Funding acquisition: Tonmoy Ghosh, Faizal Bux; Supervision: Kiran Bala, Sandhya Mishra, Faizal Bux; Writing-original draft: Tonmoy Ghosh; Writing-review and editing: Ismail Rawat, Kiran Bala, Sandhya Mishra
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ghosh, T., Rawat, I., Bala, K. et al. Assessing the potential of C-phycocyanin as a natural colorant for non-alcoholic carbonated beverages. J Appl Phycol (2024). https://doi.org/10.1007/s10811-024-03235-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10811-024-03235-9