Abstract
A local microalgal strain of Tetraselmis subcordiformis was cultivated at large-scale using open raceway pond. The temporal influence on the growth and lipidomic profile of the strain was investigated using optic density (OD) measurement and ultra-high pressure liquid chromatography. Results showed that triglycerides represented 57% of the total lipids on day 11 (exponential phase) and stayed consistently high until stationary phase, without affecting the biomass quantity. Moreover, a high expression of monounsaturated fatty acids and polyunsaturated fatty acids such as ω-3 eicosapentaenoic acid (20:5), docosahexaenoic acid (22:6), palmitic (16:0) and palmitoleic acid (16:1) was observed by stationary phase. Carotenoid analysis also revealed the increase in lutein (65.2%) and β-carotene (71.4%) from day 6 to day 15. Our study showed that T. subcordiformis contained the highest amounts of valuable lipids, fatty acids and pigments in the stationary phase, which started on day 15 of culture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ever increasing demand for food and energy due to growing world population and industrialization has resulted in the search for potential sustainable alternatives (Subhash et al. 2022). Livestock farming needs to be sustained for the production of meat due to increased global demands. The two main feeds used for livestock; corn and soybean, have become unsustainable because of climate change, land degradation, and water deprivation (Madeira et al. 2017). Thus, alternative feedstock options should be investigated to maintain livestock production.
Microalgal biomass constitutes a reservoir for various precursors, bioproducts, and value-added compounds ranging from fuel to food, such as stored lipids for biodiesel production, omega fatty acids, vitamins, proteins, and carotenoids for nutraceutical and therapeutic applications (Saadaoui et al. 2021; Siddiki et al. 2022). According to El-Ghany (2020), microalgae can be considered a promising ingredient in animal and poultry feed. n-3 long chained polyunsaturated fatty acids (PUFAs) are one of the valuable constituents produced by microalgae with a concentration of 25–38%. PUFAs such as docosahexaenoic acid (DHA, 22:6), eicosapentaenoic acid (EPA, 20:5) and α-linolenic acid (ALA, 18:3) are known for their anti inflammatory capacities, and brain and cardiovascular health benefits. These may be manufactured from many freshwater and saltwater microalgal strains.
The applications of microalgae are dictated by their metabolic behavior under cultivation conditions. Utilizing microalgal technologies for commercial applications requires enhanced production of metabolites of interest. Additonally producing multiple products from a single batch of biomass production is considered cost effective (Saadaoui et al. 2018). As a result, maximizing the utilization of microalgal biomass requires insights about various metabolite production under cultivation conditions. For instance, lipids of microalgae are of great interest as they are well exploited for essential fatty acid (FA) production (De Luca et al. 2021). However, it is equally important to identify the other potential opportunities by exploring other metabolites apart from neutral lipids, which comprise pigments (carotenoids) and therapeutically important lipids such as phospholipids, glycerolipids, sphingolipids, etc. (Chew et al. 2017).
Apart from storage, lipids mainly act as structural components of cellular membranes and even as signaling molecules (Nakamura and Li-Beisson 2016). This knowledge is used to study the ratio of structural and storage lipids in microalgae which in turn is used to induce lipid acculmulation in cells. Applying stress conditions are widely used to accelerate the stored lipids during the cultivation time (Esakkimuthu et al. 2016). However, the metabolism, regulation, and allocation of lipids vary across microalgal species. For example, the mechanisms involved in lipid metabolism vary among species such as Nannochloropsis sp. and Chlorella sp. (Martin et al. 2014). Similarly, the regulation of key enzymes involved in lipid biosynthesis varies among species under various stressors (Matich et al. 2018). Such variations ranged from storing lipids to mobilizing free FAs within the cellular organelle. Therefore, it is important to investigate the lipid regulation and metabolic responses of microalgae under various cultivation systems and conditions.
Commercialization of microalgal applications, on the other hand, is heavily reliant on the large-scale cultivation and high productivity. The large-scale systems include closed photobioreactors and open raceway ponds (ORP). Additionally, the latter has been in use for a while now, due to advantages, such as sustainability, simplicity in operation, and larger volumes (Jerney and Spilling 2020). Although they have been adopted commercially with the advantages mentioned above, there are considerable bottlenecks such as contamination by other microorganisms, lesser light penetration and shear stress due to paddle wheel (Zhang et al. 2016). Hence, finding suitable microalgal strains that are robust enough to withstand such harsh conditions in ORP is essential. Additionally, successful largescale commercialization of microalgae based products requires knowledge on how to achieve optimal growth conditions, high biomass and enriched metabolite yield and what the best time to harvest the biomass is.
Considering the above aspects, such as finding appropriate strains and cultivation conditions for large scale ORP cultivations and the vitality of lipid production in microalgae, it is important to study the microalgal lipid dynamics in ORP in time series during a cultivation period. To our knowledge this is the first report to investigate the lipid profile of microalga cultivated in an ORP time series regime, based on comprehension and review of extensive literature. In this study, insights on the best time to harvest, when large amounts of biomass and lipids are produced, are provided. The desert climate benefits the cause of microalgae by allowing for large-scale cultivation with a superior light source and lower land costs. As a result, the current study focused on time series lipidomic analysis of T. subcordiformis in a large ORP of 100,000 L in the Qatar desert climate.
Materials and method
Large scale microalgae cultivation and growth assessment
The alga used in this study is T. subcordiformis (strain number: QUCCCM50 from the Qatar University Culture Collection of Cyanobacteria and Microalgae (QUCCCM) (Saadaoui et al. 2016). A pure colony was inoculated in 10 mL of BG11 medium (Scottish Association for Marine Science 2019) prepared with seawater (40 mL sea salt in 1 L of deionized water). Culture was incubated in an illuminated shaker under an agitation of 2.5 Hz, and irradiance of 100 μmol photons m−2 s−1 with a light:dark cycle of 12:12 h and a temperature of 30 °C, corresponding to the annual average temperature in Qatar. The culture was gradually scaled up to 20 L, and then to 200 L, 1500 L, 25000L and finally 100000L ORP. All experiments were conducted in three technical replicates.
Over the experimental timespan, a 10 mL culture sample was collected daily from three different locations of the ORP to get a representative sample such as near the paddle wheel, bottom and edge of the ORP. The average optical density (OD) measurement at 750 nm was considered for the assessment of the algae growth. Guillard (1973) equation was used for the measurement of growth rate and doubling time, where:
where \({x}_{1}\) and \({x}_{2}\) are OD750nm at times t1 and t2.
The doubling time: \(dt={~}^{({\mathrm{ln}}2)}\!\left/ \!{~}_{\mu }\right.\)
Total lipid extraction and quantification
Total lipids extraction from algal biomass was carried out using the Folch et al. (1957) method with modifications of Saadaoui et al. (2016). The lipid content was determined gravimetrically, using the following equation:
Lipidomic analysis
Sample preparation of the microalgae biomass
100 mg of QUCCCM50 biomass was used for lipid extraction with chloroform and methanol (2:1). Homogenization using tissue lyser (TissueLyser II, Qiagen, USA) treatment for 5 min at 30 Hz was conducted. The organic phase was extracted and evaporated through a vacuum centrifuge. It was then reconstituted and injected into an ultra-high-pressure liquid chromatography (UHPLC) system coupled to an Orbitrap Fusion Lumos Tribrid Mass Spectrometer. The analysis was conducted in negative and positive polarity modes independently, alongside identification and quality control samples.
Data preprocessing and analysis
Identification samples were input as raw data files into the LipidSearch (version 4.2.21) database. A tolerance of 5 ppm and 10 ppm was used to check the precursor mass and fragment mass, respectively. All lipids with identified fatty acyl chains were categorized as sphingolipids, glycoglycerolipids, neutral lipids, phospholipids, and fatty acyls which is classified as Grade A identification. This data was then exported to a mass list. Compound Discoverer (version 3.1) was used to process the sample files to align and group features with a 0.2 min retention time maximum shift and 5 ppm mass tolerance. Data features which were not identified were run in the following online databases: BioCyc pathways, mzCloud, Metabolika pathways and ChemSpider (AnalytiCon Discovery, AraCyc, LipidMAPS, Indofine, Extrasynthase, Sequoia Research Products, Baoji Herbest Bio-Tech, Golm Metabolome DB, and PlantCyc). All identified compounds were then integrated by peak area for each sample.
To remove possible heteroscedasticity and correct the skewness of data distribution, a \({\mathrm{log}}2\) transformation was applied, followed by normalization and scaling. Because the experimental process can introduce nonbiological variations, data was normalized to separate biological variations. The Pareto scaling technique was used, which consists of subtracting the mean of each lipid column and dividing by the square root of its standard deviation.
Volcano plots and principal component analysis
The Volcano plot combines statistical significance and fold change differences for each unique lipid. The negative \({\mathrm{log}}10\), which is the p value from a two tailed two sample t-test, was plotted on the y-axis and the \({\mathrm{log}}2\) fold change was displayed on the x-axis. P-values for data were corrected using the Benjamini-Hochberg (BH) procedure (Benjamini and Hochberg 1995). The significance of a lipid is determined by the absolute value of the \({\mathrm{log}}2\) fold change, if it is higher than 2 and the BH corrected p value is less than 0.05, then it is significant. Significant lipids were labeled on each plot.
Principal component analysis (PCA) is a multivariate technique that aims to extract the important information from the data by replacing the original features with new ones called principal components, which are a linear combination of the original features. After running the univariate analyses (volcano) comparing each pair of groups, the data was classified into two components (multivariate) and used to create the PCA biplot.
Results
Assessment of T. subcordiformis growth and total lipid production
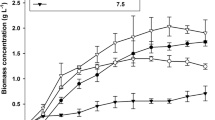
The growth of T. subcordiformis was assessed at a scale of 100,000 L using an ORP for 15 days. The sampling allowed understanding of the temporal variation in lipid content during the different growth phases (Fig. 1). QUCCCM50 had a µ and doubling time of 0.116 ± 0.02 day−1 and 6 days, respectively. Moreover there was a 28.95% increase in lipid content during the exponential phase for QUCCCM50 and the maximum lipid content was 41.75 ± 3.93%, attained on day 15.
Temporal analysis of the lipidomic profile of T. subcordiformis cultivated in open raceway pond
Temporal changes in lipid composition
To attain sufficient culture density and lipid content, lipidomic analysis was performed from day 6 onwards and observed in an incremental fashion. Among lipid classes, neutral lipids dominated, such as the triglycerides (TG) accumulation which was noted to increase up to the 11th day of cultivation period (Fig. 2). The initial assessment of day 6 revealed about 46% of TGs followed by days 11 and 15 where significant temporal increase in TG proportions of 23.9% and 19.6% was observed, respectively.
Other lipid classes observed included of phosphatidylcholine (PC), which had a slight decrease across the time series. From day 6 to day 15, reduction of PC by 20% was observed (Fig. 2). In addition to phospholipids, monogalactosyl diacyl ceramide constituted the major proportions of about 14% (day 6), which was slightly reduced to 11% on the 15th day. The remaining portion of total lipid composition was constituted by diacylglycerols, phosphatidylglycerol, phosphatidylethanolamine, phosphatidylinositol, etc. which were almost constant at each stage of analysis.
In depth lipid species analysis through volcano plot and principle component biplot
To identify the difference in the presence of significant lipids at various time intervals, lipidomic data over the experimental days was compared for high expression of lipids. The greatest difference in the expression of lipids was noted for a comparison between lipids produced by T. subcordiformis on day 15 versus day 6 (Fig. 3b). Day 15 had 34 lipids that were significantly up regulated while on day 6 only 2 lipid species were upregulated. Secondly, a high difference in expression was also noted when day 6 (4 lipids) was compared with day 11 (23 lipids) (Fig. 3a and c). It can also be important to compare the lipid expression variation between day 11 and day 15 which was 2 and 11 lipids, respectively (Fig. 3c).
PCA (Fig. 4) it showed that the first two components (67%) were needed to explain most of the data variability. The PCA biplot also evidenced more lipids for groups C and D which correspond to lipid expression on day 11 and day 15. In addition, it is worth noting that the lipid expression for day 6 was inversely proportional to day 11 while day 9 lipid profile was inversely proportional to day 15. Meaning that lipids upregulated on day 6 and day 9 were downregulated on day 11 and day 15, respectively and vice versa.
PCA Biplot for individuals and variables together, expression of lipids during the experiment. A1, A2, A3: Day 6; B4, B5, B6: Day 9; C7, C8, C9: Day 11; D10, D11, D12: Day 15. The size and color of the arrow indicate importance of the corresponding lipids and their contribution to in formation of the two dimensions respectively
Fatty acid profile of T. subcordiformis
The strain QUCCCM50 showed promising results, with high production of essential FAs when cultivated in an ORP. Although stress was not applied, over time, depletion of nitrogen and open pond conditions may have contributed to this high production. The most important lipids produced were TGs, Hex1Cer and ceramides, many of which contained desirable fatty acids such as monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs). TG, which were significantly upregulated on day 11 and day 15, contained long chain and very long chain PUFAs such as LA (18:2), ALA (18:3), EPA (20:5), and DHA (22:6) (Table 1).
Temporal influence on carotenoid production in T. subcordiformis
The production of carotenoids was investigated under different cultivation periods. Figure 5 shows the temporal influence on carotenoid production and it was confirmed that lutein and β-carotene were produced by QUCCCM50. Lutein content was relatively higher than β-carotene and both have an incremental trend across the progression of cultivation period. Lutein content remained constant during the 9th and 12th days, whereas on the 15th day, an up to 20% increase was achieved. β-carotene levels increased steadily and reached about 4.56 ± 0.12 mg. g−1DW on day 15.
Overall, carotenoids production under outdoor cultivation significantly improved in 15 days with an increase of 65.2 ± 1.8% in lutein and 71.4 ± 0.73% in β-carotene. This increase in carotenoids was significant under outdoor conditions, as overall carotenoid production accounted for about 12.56 mg g−1DW without any specific stress induction.
Discussion
In this study the growth rate of T. subcordiformis was 0.116 day−1, while in more recent studies performed on the same species, cultivated at large scale of 1500 L using ORP, found a lower growth rate and lipid content of 0.45 day−1 and 19.42% (Boopathy et al. 2020) and 0.227 day−1 and 24.2% (Patrinou et al. 2022) respectively. These results indicate variabilies in growth performace of the same algal species. It is known that the most influential factors reported to control the Tetraselmis sp. growth at outdoor scale are temperature, light intensity and photoactive radiation (Das et al. 2019). Although, Tetraselmis species have proven to flourish in varying climatic conditions (Hosseini et al. 2024) and are able to grow without external added carbon sources, pH regulation and under high solar irradiance (Zittelli et al. 2006). Overall QUCCCM50 shows very promising results when compared to other established strains, in terms of high specific growth rate and lipid content production potential without the need for growth enhancing optimizations, making it dynamic for large scale cultivation.
There have been few studies on the temporal changes in lipidomic profiling of microalgae and considerable proportions of TG in marine Tetraselmis have been reported before such by Dammak et al. (2021) who showed enhancement of TGs under nitrogen depletion conditions, whereas the present results show similar concentrations under outdoor conditions without any nutrient stress induction. The maximum TG content was achieved on the 11th day and was about 57% of the total lipids. Such temporal increase in TG can mainly be attributed to the temporal depletion of nutrients in media due to microalgal growth and eventual nutrients consumption. Once all nutrients in media are consumed, TG synthesis is enhanced as cell division slows down and the photosynthetic pathways are diverted for production of TGs and other lipid accumulation (Huerlimann et al. 2010).
In addition, it could be well understood from the results that the growth of microalgae did not reduce significantly and just entered a stationary phase on day 15, which can be suggested as an ideal time for harvesting. This can be explained by lipid accumulation without a growth retardation stage, being a preparatory phase before the growth-lipid trade-off, which would have followed eventually. However, the restriction of cultivation period to 15 days was mainly due to the aim of predicting the stage of simultaneous accumulation of biomass and triglycerides.
Other prominent lipid classes are PC and monogalactosyl diacyl ceramides MDC. While, PC act as a structural lipids for microalgal cell (Fahy et al. 2005) and are also involved in critical cellular functions, including photosynthesis and stress responses (Lu et al. 2013), MDC are sphingolipids, involved in structural functions and cellular signaling. Additionally, MDC is also used as cosmetic ingredient due to the presence of hydrocarbons and the polar head groups (Draelos 2018).
It can also be noted that the decline in phospholipids classes from day 6 to day 15 could be because of an increase in TGs, and the eventual shift of metabolic flux from structural lipid formation towards storage lipid formation. This hypothesis is supported by several reports. For example, Klok et al. (2013) investigated the simultaneous increase of TG and biomass through stress induction (excess light and nitrogen limitation), observing that the increasing concentration of TGs significantly reduced the structural lipid concentrations. Nitrogen and phosphorus limitations in Tisochrysis lutea were recently reported to increase docosahexaenoic acid (DHA 22:6) in neutral lipids, attributed to membrane polar lipid recycling, without affecting growth (Da Costa et al. 2017). The results align with the present study, except that stress was not applied. However, extending the cultivation period (15 days) would appear to result in further reduction of structural lipids and reassure the preparatory phase for T. subcordiformis's growth-lipid tradeoff. This phase is highly recommended as the best time to harvest ORP for lipid applications in the Qatar desert climate.
The PCA biplot of this study showed similar results obtained in multivariate modelling for outdoor cultivation of Tetradesmus obliquus and Graesiella emersonii by considering temperature and light, which resulted for 88% of the variance observed with two principal components (Mazzelli et al. 2020). Furthermore higher lipid content in exponential to stationary stage (Day 11-Day 15) of T. subcordiformis cultivation agree with the previous results of high lipid expression when the internalized nitrogen is depleted, and lipid pathways are activated. Moreover higher expression of TGs on day 11, which was the exponential growth phase as noted in Fig. 1, shows that the assumed lipid metabolic pathway has been initiated. According to d’Ippolito et al. (2015) when nutrients are depleted, lipid synthesis, especially production of TG is activated.
Moreover an abundance of PUFAs was noted for day 11 and day 15. These FAs have high nutritional values as humans are unable to synthesize ω-3 PUFAs (EPA and DHA) and have to receive it through food sources (Manor et al. 2019). Hence supplementing poultry feed with microalgal biomass enriched with valuable FAs can be beneficial. Although through fatty acid profiling results it can be deduced that to retrieve TGs and other lipid species with longer carbon chained ω-3 PUFAs, harvesting should be conducted earlier, as on day 11, while to harvest lipids with shorter carbon chained MUFAs, it is better to harvest on a later day as on day 15 for this strain. The reason may be that the initial lipid pathways have higher production of longer chain fatty acid lipids while as growth slows down, shorter chained fatty acids are assembled.
Pigments obtained from microalgae can be high value products with potential of being commercialized, hence they were investigated as well. Carotenoid pigments are well known antioxidants valued for their structural and functional importance in photosynthesis especially for light harvesting and photoprotective functionalities (Haoujar et al. 2019). Carotenoids also have an array of functions such as anti-diabetic, anti-angiogenic, anti-inflammatory, anti-cancer, anti-obesity, anti-oxidant properties, and cardio-protective, which potentially grab the greater interest of pharmaceutical sectors (Sathasivam and Ki 2018; Saadaoui et al. 2020). In addition, the natural color and nutritional properties of carotenoids have wider applications in the food and feed industries (Kim 2015).
Furthermore the more abundant carotenoid, lutein, is well known for its potential in lowering cardiovascular diseases and can be beneficial for eye diseases and overall eye function, as well as for adult cognitive health (Moran et al. 2018). Feed based benefits of microalgal pigments have also been highlighted in previous studies. It was found that supplementing brown marine algae Sargassum dentifebium to hens increased the total carotene in eggs and reduced the plasma and yolk cholesterol levels (Al-Harthi and El-Deek 2012). In another study Chlorella sp. was added to feed of chickens and carotenoids such as lutein, β-carotene and zeaxanthin were deposited in yolks in high concentration boosting egg yolk colour (Kotrbáček et al. 2013). Overall, adding microalgal biomass containing carotenoids to broiler feed can enhance the quality of eggs and indirectly have a beneficial impact on human health.
The aim of this study was to analyze the lipid profile of T. subcordiformis in an ORP over cultivation time. Growth, lipidomic profile and carotenoid production was investigated. Throughout the cultivation time, which was 15 days, it was found that the exponential phase, evidenced on day 11 had the highest amount of lipids with mostly triglycerides. The fatty acid profile of the strain was rich as well and so was the carotenoid. Fatty acids such as EPA and DHA were found abundantly in addition to carotenoids β-carotene and lutein by the end of culture. According to this study the best time to harvest T. subcordiformis biomass with high value products would be the end of culture which is day 15, the stationary phase. As the stationary phase of a strain is important for harvesting enriched biomass, for large scale cultivation, it is important to investigate the growth cycle, which varies from strain to strain.
Data availability
The data generated will be made available by the corresponding author upon reasonable request.
References
Al-Harthi MA, El-Deek AA (2012) Effect of different dietary concentrations of brown marine algae (Sargassum dentifebium) prepared by different methods on plasma and yolk lipid profiles, yolk total carotene and lutein plus zeaxanthin of laying hens. Ital J Anim Sci 11:e64
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57:289–300
Boopathy AB, Jayakumar T, Chinnasamy S, Rajaram MG, Mohan N, Nagaraj S, Rengasamy R, Manubolu M, Sheu JR, Chang CC (2020) Biomass and lipid production potential of an Indian marine algal isolate Tetraselmis striata BBRR1. Energies 13:341
Chew KW, Yap JY, Show PL, Suan NH, Juan JC, Ling TC, Lee DJ, Chang JS (2017) Microalgae biorefinery: High value products perspectives. Bioresour Technol 229:53–62
Da Costa F, Le Grand F, Quéré C, Bougaran G, Cadoret JP, Robert R, Soudant P (2017) Effects of growth phase and nitrogen limitation on biochemical composition of two strains of Tisochrysis lutea. Algal Res 27:177–189
Dammak M, Hlima H, Ben Elleuch F, Pichon C, Michaud P, Fendri I, Abdelkafi S (2021) Flow cytometry assay to evaluate lipid production by the marine microalga Tetraselmis sp. using a two stage process. Renew Energy 177:280–289
Das P, Thaher M, AbdulQuadir M, Khan S, Chaudhary A, Al-Jabri H (2019) Long-term semi-continuous cultivation of a halo-tolerant Tetraselmis sp. using recycled growth media. Bioresour Technol 276:35–41
De Luca M, Pappalardo I, Limongi AR, Viviano E, Radice RP, Todisco S, Martelli G, Infantino V, Vassallo A (2021) Lipids from microalgae for cosmetic applications. Cosmetics 8:52
Draelos ZD (2018) The science behind skin care: Moisturizers. J Cosmet Dermatol 17:138–144
d’Ippolito G, Sardo A, Paris D, Vella FM, Adelfi MG, Botte P, Gallo C, Fontana A (2015) Potential of lipid metabolism in marine diatoms for biofuel production. Biotechnol Biofuels 8:28
El-Ghany WAA (2020) Microalgae in poultry field: A comprehensive perspectives. Adv Anim Vet Sci 8:888–897
Esakkimuthu S, Krishnamurthy V, Govindarajan R, Swaminathan K (2016) Augmentation and starvation of calcium, magnesium, phosphate on lipid production of Scenedesmus obliquus. Biomass Bioenerg 88:126–134
Fahy E, Subramaniam S, Brown HA, Glass CK, Merrill AH, Murphy RC, Raetz CRH, Russell DW, Seyama Y, Shaw W (2005) A comprehensive classification system for lipids. J Lipid Res 46:839–861
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Guillard RRL (1973) Division rates. In: Stein JR (ed) Handbook of phycological methods: Culture methods and growth measurements. Cambridge University Press, Cambridge, pp 289–311
Haoujar I, Cacciola F, Abrini J, Mangraviti D, Giuffrida D, Oulad El Majdoub Y, Kounnoun A, Miceli N, Fernanda Taviano M, Mondello L (2019) The contribution of carotenoids, phenolic compounds, and flavonoids to the antioxidative properties of marine microalgae isolated from Mediterranean Morocco. Molecules 24:4037
Hosseini, H, Saadaoui, I, Cherif, M, Siddiqui, SA, Sayadi, S (2024) Exploring the dynamics of algae-associated microbiome during the scale up process of Tetraselmis sp. microalgae: a metagenomics approach. Bioresour Technol 393:129991
Huerlimann R, de Nys R, Heimann K (2010) Growth, lipid content, productivity, and fatty acid composition of tropical microalgae for scale-up production. Biotechnol Bioeng 107:245–257
Jerney J, Spilling K (2020) Large scale cultivation of microalgae: Open and closed systems. Meth Mol Biol 1980:1–8
Kim SK (ed) (2015) Handbook of marine microalgae: Biotechnology advances. Academic Press, Amsterdam
Klok AJ, Martens DE, Wijffels RH, Lamers PP (2013) Simultaneous growth and neutral lipid accumulation in microalgae. Bioresour Technol 134:233–243
Kotrbáček V, Skřivan M, Kopecký J, Pěnkava O, Hudečková P, Uhríková L, Doubek J (2013) Retention of carotenoids in egg yolks of laying hens supplemented with heterotrophic Chlorella. Czech J Anim Sci 58:193–200
Lu S, Wang J, Ma Q, Yang J, Li X, Yuan YJ (2013) Phospholipid metabolism in an industry microalga Chlorella sorokiniana: the impact of inoculum sizes. PLoS One 8:e70827
Madeira MS, Cardoso C, Lopes PA, Coelho D, Afonso C, Bandarra NM, Prates JA (2017) Microalgae as feed ingredients for livestock production and meat quality: A review. Livest Sci 205:111–121
Manor ML, Derksen TJ, Magnuson AD, Raza F, Lei XG (2019) Inclusion of dietary defatted microalgae dose-dependently enriches ω-3 fatty acids in egg yolk and tissues of laying hens. J Nutr 149:942–950
Martin GJO, Hill DRA, Olmstead ILD, Bergamin A, Shears MJ, Dias DA, Kentish SE, Scales PJ, Botté CY, Callahan DL (2014) Lipid profile remodeling in response to nitrogen deprivation in the microalgae Chlorella sp. (Trebouxiophyceae) and Nannochloropsis sp. (Eustigmatophyceae). PLoS One 9:e103389
Matich EK, Ghafari M, Camgoz E, Caliskan E, Pfeifer BA, Haznedaroglu BZ, Atilla-Gokcumen GE (2018) Time-series lipidomic analysis of the oleaginous green microalga species Ettlia oleoabundans under nutrient stress. Biotechnol Biofuels 11:29
Mazzelli A, Cicci A, Di Caprio F, Altimari P, Toro L, Iaquaniello G, Pagnanelli F (2020) Multivariate modeling for microalgae growth in outdoor photobioreactors. Algal Res 45:101663
Moran NE, Mohn ES, Hason N, Erdman JW, Johnson EJ (2018) Intrinsic and extrinsic factors impacting absorption, metabolism, and health effects of dietary carotenoids. Adv Nutr 9:465–492
Nakamura Y, Li-Beisson Y (eds) (2016) Lipids in plant and algae development. Springer, Cham
Patrinou V, Daskalaki A, Kampantais D, Kanakis DC, Economou CN, Bokas D, Kotzamanis Y, Aggelis G, Vayenas DV, Tekerlekopoulou AG (2022) Optimization of cultivation conditions for Tetraselmis striata and biomass quality evaluation for fish feed production. Water 14:3162
Saadaoui I, Al Ghazal G, Bounnit T, Al Khulaifi F, Al Jabri H, Potts M (2016) Evidence of thermo and halotolerant Nannochloris isolate suitable for biodiesel production in Qatar culture collection of cyanobacteria and microalgae. Algal Res 14:39–47
Saadaoui I, Bounnit T, Muraikhi M, Rasheed R, Alghasal G, Al Jabri H (2018) Improvement of both lipid and biomass productivities of Qatar Chlorocystis isolate for biodiesel production and food security. Phycol Res 66:182–188
Saadaoui I, Rasheed R, Abdulrahman N, Bounnit T, Cherif M, Al Jabri H, Mraiche F (2020) Algae-derived bioactive compounds with anti-lung cancer potential. Mar Drugs 18:197
Sathasivam R, Ki JS (2018) A review of the biological activities of microalgal carotenoids and their potential use in healthcare and cosmetic industries. Mar Drugs 16:26
Saadaoui I, Rasheed R, Aguilar A, Cherif M, Al Jabri H, Sayadi S, Manning SR (2021) Microalgal-based feed: Promising alternative feedstocks for livestock and poultry production. J Anim Sci Biotechnol 12:76
Scottish Association for Marine Science (2019) BG11 (Blue-Green Medium) Freshwater algae and protozoa. Culture Collection of Algae and Protozoa. https://www.ccap.ac.uk/wp-content/uploads/MR_BG11.pdf; accessed on 15 February 2021
Siddiki SYA, Mofijur M, Kumar PS, Ahmed SF, Inayat A, Kusumo F, Badruddin IA, Khan TMY, Nghiem LD, Ong HC (2022) Microalgae biomass as a sustainable source for biofuel, biochemical and biobased value-added products: An integrated biorefinery concept. Fuel 307:121782
Subhash GV, Rajvanshi M, Kumar GRK, Sagaram US, Prasad V, Govindachary S, Dasgupta S (2022) Challenges in microalgal biofuel production: A perspective on techno economic feasibility under biorefinery stratagem. Bioresour Technol 343:126155
Zhang TY, Hu HY, Wu YH, Zhuang LL, Xu XQ, Wang XX, Dao GH (2016) Promising solutions to solve the bottlenecks in the large-scale cultivation of microalgae for biomass/bioenergy production. Renew Sust Energy Rev 60:1602–1614
Zittelli GC, Rodolfi L, Biondi N, Tredici MR (2006) Productivity and photosynthetic efficiency of outdoor cultures of Tetraselmis suecica in annular columns. Aquaculture 261:932–943
Acknowledgements
We also thank the team members of the Centre for Sustainable Development, Qatar University for their continued help. The Open access was funded by Qatar National Library.
Funding
Open Access funding provided by the Qatar National Library. This publication was made possible by the MME grant # [MME 01–0924-190063] from the Qatar National Research Fund (a member of Qatar Foundation).
Author information
Authors and Affiliations
Contributions
Imen Saadaoui: Conceptualization, Supervision, Methodology, writing—review and editing and funding acquisition; Maroua Cherif: Investigation, Data acquisition, Methodology, writing—review and editing; Simil Amir Siddiqui: writing—original draft preparation, writing—review and editing, data interpretation; Sivakumar Esakkimuthu; writing—original draft preparation; Mohammed AbdulQuadir: Data acquisition; Mohamad El Anbari: Formal Analysis; Sami Sayadi: writing—review and editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saadaoui, I., Cherif, M., Siddiqui, S.A. et al. Unveiling Tetraselmis subcordiformis lipidome dynamics during large-scale cultivation in open raceway pond. J Appl Phycol 36, 1125–1134 (2024). https://doi.org/10.1007/s10811-023-03166-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-03166-x