Abstract
The mitigation of coral bleaching requires adaptation of its endosymbionts to rising ocean temperatures, acidity, and ultra-violet radiation. While genetic transformation may offer a means for enhancing endosymbiont resilience to these environmental stressors, the opportunity for doing so in dinoflagellates is likely hampered, in part, by their armored cortex, which may present a formidable barrier to intracellular delivery. Here, using Breviolum minutum (Clade B Symbiodiniaceae) as a model, we show that this barrier may be moderated by first disrupting the outer plasma membrane with the detergent octyl β-D-glucopyranoside, followed by enzymatic digestion of the underlying cellulose with Cellulase RS and Macerozyme R-10. Treatment using this new protocol results in 61% reduction in calcofluor-based cell wall staining (i.e., 25% staining for protocol vs. 64% for control), thus demonstrating the ability for considerable cell wall digestion. Furthermore, protoplasts isolated thereby exhibit rapid cell wall recovery, as well as comparable PSII activity and cell growth as the control, thus suggesting that the protocol minimally affects acute- and long-term cellular function. Finally, the isolated protoplasts also show a potentially slight increase in permeability to a model exogenous cargo after electroporation with a non-optimized protocol. Collectively, this constitutes the first known successful generation of viable B. minutum protoplasts, and thus, serves as a foundation for future studies seeking to lower the barrier for transformation of these and possibly other Symbiodiniaceae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The survival of the ocean’s coral reefs is dependent on the health of their endosymbiotic dinoflagellate microalgae in the family Symbiodiniaceae, which serve as the primary source of the coral’s essential nutrients (Hoegh-Guldberg 1999; Davy et al. 2012). Rising ocean temperatures and acidification have led to hostile conditions for Symbiodiniaceae resulting in death and expulsion from corals, and consequently, numerous coral bleaching events worldwide (Hughes et al. 2017, 2018; Eakin et al. 2019; Starko et al. 2023). To mitigate this, assisted evolution has been proposed as a means of generating desirable traits in Symbiodiniaceae, such as higher tolerance for heat and acidity (van Oppen et al. 2017; Buerger et al. 2020; Howells et al. 2021). Combined with chemical and UV-induced random mutagenesis, targeted mutagenesis via genetic transformation would help accelerate the process of obtaining resilient strains, and also facilitate studies seeking to advance understanding of the underlying cellular mechanisms relevant to bleaching triggers (Douglas 2003; Levin et al. 2017b; Jinkerson et al. 2022).
To achieve transformation, foreign genetic constructs must first be introduced into the cell, often via the temporary disruption of the cell wall to provide a transport pathway for intracellular delivery. For most marine algae, this is easily achieved using a variety of techniques, including biolistic particle bombardment, silicon carbide whisker or glass bead abrasion, Agrobacterium-mediated delivery, or electroporation of isolated protoplasts generated by enzymatic digestion of the cellulosic cell wall (Potrykus and Shillito 1986; Davey et al. 2010; Qin et al. 2012). However, Symbiodiniaceae have proven to be far more recalcitrant to genetic transformation, and as such, there have been few reports in this regard. Moreover, for those that have reported success, extremely low transformation efficiencies have been observed (Gornik et al. 2022), as well as low motility (Lohuis and Miller 1998), and impaired photosynthetic activity and cell division (Ortiz-Matamoros et al. 2015). Finally, attempts to reproduce some of these studies have been unsuccessful (Chen et al. 2019).
One cause of the recalcitrance of Symbiodiniaceae may be the potential barrier to intracellular delivery that is imposed by its unique armored cortex. While dependent upon a myriad of factors, (e.g., strain, life cycle stage, life history, environment, etc.), this complex multilayered structure often consists of an outermost membrane that overlies various components, including vesicle-encased cellulosic thecal plates. (Morrill and Loeblich 1983; Spector 1984; Wakefield et al. 2000; Kwok et al. 2023). This may also hamper access to the cellulosic components, thus constraining the opportunity for using conventional enzymatic protoplasting techniques to improve intracellular access (Levin et al. 2017a). The first known method for the protoplast isolation of Symbiodiniaceae (Clades A and C, specifically) was established by Levin et al. (2017a), and later refined through optimization of incubation time, as well as the addition of a macerating enzyme (Bashir et al. 2022). However, the ultimate utility of these methods may be limited by their adverse impact on cellular function, which results from the need for use of long enzyme incubation times to bypass the transport barriers imposed by the various membranous structures.
Here, we report the adaptation of these earlier methodologies towards the first generation of protoplasts from another Symbiodiniaceae strain, Breviolum minutum (formerly known as Symbiodinium minutum, or Clade B). Key to this new protocol’s success is the use of an initial brief exposure to a detergent to permeabilize the outer membrane and thus improve enzyme access to the underlying cellulosic cell wall components. Using a combination of fluorescence microscopy and flow cytometry, we show that this new protocol enables considerable cell wall digestion, as well as rapid cell wall recovery. Moreover, using Photosystem II (PSII) measurements and long-term culture, we also show that the resulting protoplasts maintain comparable photosynthetic activity and cell growth as untreated controls. Finally, using a non-optimized protocol for electroporation-mediated delivery of fluorescein isothiocyanate (FITC) conjugated dextran, we show a potentially slight improvement in intracellular delivery. In doing so, this work represents a crucial first step towards addressing the limited intracellular accessibility of B. minutum specifically (Bashir et al. 2022), and potentially other Symbiodiniaceae more broadly.
Materials and methods
Cell culture and maintenance
Marine Broth (MB) was prepared by dissolving BD Difco Dehydrated Culture Media: Marine Broth 2216 (Fisher Scientific) in deionized water (37.4 g L−1), and the medium was sterilized by autoclaving. Breviolum minutum strain SSB01 (Xiang et al. 2013) was maintained in MB under 16 h light:8 h dark conditions at an irradiance of 73.56 µmol photons m−2 s−1 and passaged every two weeks into sterile flasks capped with silicone sponge closures at 15 × 106 cells per 75 mL MB.
Cell wall digestion conditions and controls
Treatment conditions for cell wall digestion were adapted from Levin et al. (2017a, b) and Bashir et al. (2022), with an additional step for outer membrane permeabilization with the detergent octyl β-D-glucopyranoside, and slight adjustments to enzyme media composition. As illustrated in Fig. 1, 107 B. minutum cells from 2-week-old cultures were centrifuged at 2000 ×g and resuspended in 10 mL sterile MB at pH 7.0 containing 5 mM octyl β-D-glucopyranoside (Sigma-Aldrich Inc.). The suspension was transferred to a 25 mL sterile Erlenmeyer flask, sealed with aluminum foil and parafilm, and incubated in a shaker (VWR Incubated Shaker, 120 V, Cat No. 76407–112) at 100 rpm and 30 °C for 1 h. Cells were pelleted and the supernatant was replaced with 10 mL of a MB solution containing 0.5 M D-sorbitol (Sigma-Aldrich Inc.), 3% cellulase RS (GoldBio), and 0.75% macerozyme R-10 (GoldBio), pH 5.0 and sterilized by filtration with 0.22 µm nylon filters. The suspension was transferred to a flask and incubated in a shaker at 100 rpm and 30 °C for 18 h. Digested cells were centrifuged at 200 ×g and resuspended in sterile MB at pH 7.0 containing 0.5 M D-sorbitol and 0.025 M calcium chloride dihydrate (Spectrum Chemical Mfg. Corp.). After three washes, cells were resuspended into the same media at a final volume of 15 mL and then transferred to sterile flasks with breathable silicone sponge closures for long-term storage under 16 h light:8 h dark conditions. In addition to the treatment condition described above, which will be referred to as ‘ENZ + OG’, three additional treatment groups were prepared concurrently: cells treated with octyl β-D-glucopyranoside only (‘OG’); cells treated with cellulase RS and macerozyme R-10 only (‘ENZ’); and cells treated with neither (‘Control’). These samples were otherwise processed identically to the ENZ + OG treatment group (Table 1).
Fluorescence microscopy
One million cells were resuspended in 1 mL Hank’s Balanced Salt Solution (HBSS, Sigma-Aldrich Inc.), and then stained for 10 min with 0.52 mM fluorescent brightener 28 disodium salt solution (Sigma-Aldrich Inc.), also known as calcofluor white M2R (CFW). Cells were then washed with fresh HBSS and aliquoted onto microscope slides with cover slips for oil-immersion imaging (Revolve Hybrid Fluorescence Microscope, Echo). Cell wall fluorescence was captured with the DAPI filter (Ex: 380/30, Em: 450/50), and cell autofluorescence was captured with the CY5 filter (Ex: 630/40, Em: 700/75). The exposure time (20 ms), gain (low), and levels (default) were held constant across all collected micrographs.
Flow cytometry
Flow cytometry was conducted to measure fluorescence intensities from 52 nM CFW and chlorophyll autofluorescence in the cell wall removal studies (Ex: 405, Em: 445/45 and Ex: 561, Em: 695/40, respectively), and FITC-Dextran in the delivery studies (Ex: 488, Em: 530/30) (NovoCyte Flow Cytometer, Agilent). Data analysis was performed using a commercial software package (FCS Express, De Novo Software). The percentage of CFW-stained cells was determined by gating based on exclusion of debris and 99% of the unstained control population (Supplementary Information Fig. S1). Chlorophyll autofluorescence intensity was determined by gating out debris, then using the native histogram statistics feature in the analysis software to extract the median fluorescence intensity (Fig. S2). The percentage of FITC-stained cells was determined by gating based on exclusion of debris and 99% of the untreated control population (Fig. S3).
Confocal microscopy
After staining 106 cells with 0.52 mM CFW for 10 min in 1 mL HBSS, cells were washed with fresh HBSS and aliquoted onto microscope slides with cover slips for oil-immersion imaging (TCS SP5 Confocal Microscope, Leica Microsystems). Cell wall fluorescence was captured using a 405 nm 5.5 mW solid state laser at 20% power, 415 – 435 nm emission window, PMT detector, and 700 V gain. Cell autofluorescence was captured using a 633 nm 4 mW helium neon laser at 3% power, 675 – 685 nm emission window, hybrid detector, and 100% gain. All parameters were held constant across all collected micrographs.
Photosystem II measurements
Approximately one million cells were resuspended in 1 mL HBSS, and then dark-adapted for 1 h prior to the acquisition of minimum (F0) and maximum (Fm) fluorescence (AquaPen-C AP 110-C, Photon Systems Instruments). The PSII activity (Fv/Fm) was calculated using Eq. 1.
Cell growth log
Immediately following the cell wall digestion protocol, 3 mL aliquots of each treatment group were cultured in 15 mL of sterile MB solution at pH 7.0 containing 0.5 M D-sorbitol and 0.025 M calcium chloride dihydrate. The concentrations of cultured protoplasts and control cells were acquired on days 0, 1, 3, 5, 14, 21, and 28 via hemacytometer cell counting (#3200, Hausser Scientific).
FITC-dextran delivery via electroporation
Proof-of-principle intracellular delivery studies were performed using a protocol reported previously for transformation of walled B. minitum cells (i.e., non-protoplasted) (Gornik et al. 2022), which utilized electroporation process parameters originally derived by the instrument manufacturer for the parasitic protozoan Perkinsus marinus (Fernández-Robledo et al. 2008; Sakamoto et al. 2022). One million freshly isolated protoplasts or control cells were centrifuged at 200 ×g and resuspended into 100 µL electroporation media (Nucleofector Basic Solution for Parasites + Supplement 1, Lonza) containing 125 µM 3000 – 5000 Da FITC-Dextran (Sigma-Aldrich Inc.). The suspension was electroporated (Program D-023, Nucleofector 2b, Lonza), and the samples were immediately transferred to 2 mL of MB at pH 7.0 containing 0.5 M D-sorbitol and 0.025 M calcium chloride dihydrate. The samples were allowed to recover for 30 min and were then analyzed by flow cytometry. In addition to ENZ + OG treated cells electroporated with FITC-dextran, other samples included control cells without treatment, control cells electroporated in the presence of FITC-dextran, ENZ + OG cells without treatment, ENZ + OG cells exposed to FITC-dextran without electroporation, and ENZ + OG cells electroporated in the absence of FITC-dextran. Viability was measured by staining with 10 µM fluorescein diacetate (FDA) for 10 min, followed by washing with HBSS and observation via fluorescence microscopy using the FITC filter (Ex: 470/40 Em: 525/50).
Statistical analyses
All cell wall degradation studies were conducted in triplicate, and statistical analyses were performed using the t-Test: Paired Two Sample for Means (Excel, Microsoft). The intracellular delivery and simultaneous exposure studies were performed in singlicate.
Results
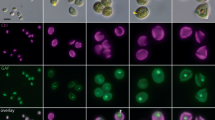
Representative fluorescence micrographs illustrating the impact of the various treatment conditions on cell wall staining, cell morphology, and chlorophyll autofluorescence are shown in Fig. 2a (complementary confocal micrographs are shown in Figs. S4 and S5). Minimal CFW cell wall staining is observed for the ENZ + OG condition, and a subtle reduction in cell size is observed, indicative of increased sensitivity to high osmolarity in protoplasts, as also seen in a larger survey (Fig. S6). Autofluorescence imaging suggests a subtle redistribution and reduction of chlorophyll intensity for the ENZ + OG condition, the latter of which is also seen at the population scale (Fig. S7). Additionally, the micrographs in Fig. 2a show that the enzymes alone (ENZ) also reduce CFW staining, but to a lesser extent than the ENZ + OG condition. Finally, the micrographs show that the detergent alone (OG) minimally affects CFW staining.
Fluorescence-based characterization of cell wall removal. a Representative fluorescence micrographs for the control, detergent-only (OG), enzymes-only (ENZ), and enzymes + detergent (ENZ + OG) treatment conditions are shown. Top row: bright-field images. Second row: calcofluor white M2R (CFW) cell wall stain fluorescence. Third row: chlorophyll autofluorescence. Bottom row: overlay of CFW fluorescence, bright-field, and autofluorescence. Scale bar = 2 µm. b Flow cytometry histograms depicting the distribution of CFW fluorescence intensities for each treatment condition. c Percentage of CFW-stained cells for each treatment condition (mean ± SE, n = 3). ** p < 0.01; *** p < 0.001
The flow cytometry studies corroborate the CFW fluorescence trends observed by microscopy. The histogram for the ENZ + OG condition skews heavily towards the unstained control (Fig. 2b), thus indicating that significant cell wall removal has occurred. However, there is also a small shoulder that skews towards the stained control, which indicates that a small population of cells remains with sufficient cellulosic content for measurable CFW staining. The enzymes alone (ENZ) also produce a bimodal distribution, but to a lesser extent than the ENZ + OG condition, and the detergent alone (OG) mirrors the stained control. Figure 2c further quantifies the impact of the various treatments on CFW fluorescence. A 61% reduction in the percentage of CFW-stained cells is observed for the ENZ + OG condition compared to the stained control (25% ± 0.58% CFW + cells for ENZ + OG vs. 64.33% ± 2.85% for stained control; mean ± SE). The enzymes alone (ENZ) reduce the number of CFW-stained cells to a lesser extent, and the detergent alone (OG) has minimal impact.
Figure 3 depicts representative confocal micrographs for the ENZ + OG condition taken over the course of a few days after digestion. CFW fluorescence is observed to be largely absent immediately following digestion (day 0); however, it begins to return at day 1. Chlorophyll autofluorescence appears slightly diminished at day 0, but quickly recovers as well.
Fluorescence-based characterization of cell wall regeneration after protoplast isolation. Representative confocal micrographs prior to cell wall digestion, and post-digestion up to two days after the enzymes + detergent (ENZ + OG) treatment are shown. Top row: CFW fluorescence. Middle row: chlorophyll autofluorescence. Bottom row: overlay of CFW fluorescence and autofluorescence. Scale bar = 2 µm
Figure 4a portrays the impact of the various treatment conditions on PSII activity. There appears to be a slight reduction in average PSII activity for the ENZ + OG condition compared to the untreated control (0.36 ± 0.04 vs. 0.41 ± 0.03, mean ± SE), although the difference is not statistically significant. The relatively low PSII activities observed across all conditions may arise from their extended exposure to elevated temperatures, which is known to reduce PSII activity in B. minutum (Bayliss et al. 2019). The impact of the various treatment conditions on long-term cell growth is shown in Fig. 4b. Growth for all treatment conditions is largely consistent with the untreated control.
Cellular function after protoplast isolation. a Average Photosystem II activity after 1 h dark incubation following the control, detergent-only (OG), enzymes-only (ENZ), and enzymes + detergent (ENZ + OG) treatment conditions (mean ± SE, n = 3). * p < 0.05. b Cell growth after each treatment condition (mean ± SE, n = 3)
Figure 5a illustrates the impact of the ENZ + OG treatment on electroporation-mediated intracellular delivery of FITC-dextran. A subtle, yet non-negligible sub-population is observed in the positive green fluorescence region for ENZ + OG: FITC + Electroporation condition. This sub-population is not seen in the control, nor any of the other conditions, thus suggesting it is indicative of intracellular delivery, although further replicates are required for confirmation. Representative fluorescence micrographs of FDA-based viability staining of control and ENZ + OG cells are shown in Fig. 5b, before and after electroporation. FDA staining is largely absent for the ENZ + OG condition for the freshly isolated protoplasts (“Before electroporation”), as well as immediately after electroporation (Day 0). However, FDA staining comparable to the controls returns by day 2.
Proof-of-principle intracellular delivery attempt of model cargo (FITC-dextran). a Flow cytometry histograms depicting the distribution of green fluorescence intensities from the control and the enzymes + detergent (ENZ + OG) treatment condition following electroporation. The arrow highlights the presence of a small sub-population in the ENZ + OG: FITC + Electroporation condition that could be indicative of FITC delivery. b Fluorescein diacetate (FDA) viability staining of control and ENZ + OG treated cells, before and after electroporation. Scale bar = 40 µm
Discussion
The cell wall digestion protocol detailed herein is, to the best of our knowledge, the first to successfully generate B. minutum protoplasts. Our fluorescence imaging and flow cytometry studies (Figs. 2, S4, & S5) provide clear evidence of its potential for achieving significant wall removal (61% reduction of CFW + cells compared to untreated control). This is corroborated by the observation that the ENZ + OG treated cells also display greater tonicity sensitivity than untreated control cells (Fig. S6), presumably due to their diminished cell wall rigidity. Similar sensitivity has been reported for protoplasts generated from other Symbiodiniaceae strains (Levin et al. 2017a).
Importantly, our studies also suggest that the ENZ + OG protocol minimally impacts cellular function. Although photosynthetic activity in all samples were lower (~ 0.4) than typically observed for B. minutum (~ 0.5–0.6), this may be attributed to prolonged exposure to elevated temperatures, a known factor that can affect PSII activity in B. minutum (Bieri et al. 2016; Bayliss et al. 2019; Marinov et al. 2021). However, in light of the protoplasts’ comparable PSII activity to that of the untreated control (Fig. 4a), the rapid cell wall recovery (Fig. 3), and cell growth consistent with the untreated control (Fig. 4b), it is evident that cells were able to retain their overall health. As discussed earlier, the success of ENZ + OG protocol lies in the opportunity that the initial membrane permeabilization step provides for reducing enzyme incubation duration, and thus, the adverse impact thereof on cellular function. Interestingly, recent studies (Bashir et al. 2022) have shown that careful control of protoplasting conditions within a microfluidic system may also reduce the impact of protoplasting on cellular function. However, while such systems provide new means for studying and optimizing the protoplasting process at the single-cell level, their ultimate utility for routine processing of bulk samples is uncertain, since it is unclear whether they could be scaled to sufficient throughputs.
Our proof-of-principle electroporation studies also suggest that the ENZ + OG protocol may provide eventual promise for improving intracellular delivery (Fig. 5a). While the present data do not definitively demonstrate delivery, especially given the rather modest increase in FITC positive cells observed over the control (~ 1.1%, Fig. S3), it is important to emphasize that the electroporation process parameters used in this study were not optimized for dinoflagellates. Moreover, using the same electroporation protocol, Gornik et al. (2022) were only able to achieve 0.1% efficiency for transient GFP expression from a plasmid vector in walled B. minutum cells. Although direct comparison with our results is not possible, this does suggest that delivery has been achieved in the present study as well. Finally, while the absence of FDA staining in the isolated protoplasts in Fig. 5b, both before and after electroporation, would suggest diminished viability thereof, this is likely an artifact resulting from their increased permeability, which would hinder intracellular retention of the hydrolyzed fluorescein. This conjecture is supported by the comparable photosynthetic activity seen for the ENZ + OG condition vs. control (Fig. 4a), as well as the rapid return of FDA staining for the ENZ + OG condition at day 2.
While OG has not been previously explored for use in the permeabilization of dinoflagellate membranes for protoplast isolation, its selection herein was motivated by a number of factors. For example, alkyl glucosides, in general, are known for their gentle surfactant properties, and they are commonly employed to solubilize proteins through the disruption and/or integration within membrane lipid bilayers, consequently modifying permeability (Paternostre et al. 1988; Seddon et al. 2004; Dinesh et al. 2018). Moreover, OG was selected specifically for its amphiphilicity, ability to intercalate into membranes, and high critical micelle concentration (19—25 mM), the latter of which allows use of concentrations that are sufficient to achieve permeabilization without the formation of micelles, thus simplifying removal by washing (Dinesh et al. 2018).
Finally, it is important to note that the use of sequential detergent and enzyme exposure may also be critical to success. In our earlier exploratory pathfinding efforts, simultaneous exposure to enzymes and detergents was observed to yield significant cell wall removal (Figs. S8 & S9), but this came at the expense of cell growth and PSII activity (Fig. S10). This suggested that simultaneous exposure may lead to excessive permeabilization of interior membranes (e.g., cytoplasmic membrane), thus motivating the development of the sequential exposure protocol, as well as minor adjustment of the detergent and enzyme concentrations used therein.
In conclusion, we have demonstrated that the addition of an initial detergent-induced membrane permeabilization step enables efficient protoplasting of B. minutum, for the first time, while also maintaining cellular function. This therefore motivates future studies seeking to further optimize the protoplasting conditions, better understand the impact thereof on cell wall structure (e.g., transmission electron microscopy studies to confirm the extent of digestion and identify potential structural alterations), and explore the opportunity for extension to other Symbiodiniaceae strains. Furthermore, the observation of a potentially slight enhancement of FITC-dextran delivery efficiency using a non-optimal electroporation protocol suggests potential for further optimization and improvement. This work therefore serves as a valuable starting point for future studies seeking to genetically transform B. minutum and possibly other Symbiodiniaceae strains that possess similar amphiesmal structures.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Bashir F, Kovács S, Ábrahám Á, Nagy K, Ayaydin F, Valkony-Kelemen I, Ferenc G, Galajda P, Tóth SZ, Sass L, Kós PB, Vass I, Szabó M (2022) Viable protoplast formation of the coral endosymbiont alga Symbiodinium spp. in a microfluidics platform. Lab Chip 22:2986–2999
Bayliss SLJ, Scott ZR, Coffroth MA, terHorst CP (2019) Genetic variation in Breviolum antillogorgium, a coral reef symbiont, in response to temperature and nutrients. Ecol Evol 9:2803–2813
Bieri T, Onishi M, Xiang T, Grossman AR, Pringle JR (2016) Relative contributions of various cellular mechanisms to loss of algae during cnidarian bleaching. PLoS One 11:e0152693
Buerger P, Alvarez-Roa C, Coppin CW, Pearce SL, Chakravarti LJ, Oakeshott JG, Edwards OR, van Oppen MJH (2020) Heat-evolved microalgal symbionts increase coral bleaching tolerance. Sci Adv 6:eaba2498
Chen JE, Barbrook AC, Cui G, Howe CJ, Aranda M (2019) The genetic intractability of Symbiodinium microadriaticum to standard algal transformation methods. PLoS One 14:e0211936
Davey MR, Anthony P, Patel D, Power JB (2010) Plant Protoplasts: Isolation, Culture and Plant Regeneration. In: Davey MR, Anthony P (eds) Plant Cell Culture: Essential Methods. John Wiley & Sons, Ltd, Chichester, UK, pp 153–173
Davy SK, Allemand D, Weis VM (2012) Cell biology of cnidarian-dinoflagellate symbiosis. Microbiol Mol Biol Rev 76:229–261
Dinesh M, Deepika S, HarishKumar R, Selvaraj CI, Roopan SM (2018) Evaluation of octyl-β-D-Glucopyranoside (OGP) for cytotoxic, hemolytic, thrombolytic, and antibacterial activity. Appl Biochem Biotechnol 185:450–463
Douglas AE (2003) Coral bleaching––how and why? Mar Pollut Bull 46:385–392
Eakin CM, Sweatman HPA, Brainard RE (2019) The 2014–2017 global-scale coral bleaching event: insights and impacts. Coral Reefs 38:539–545
Fernández-Robledo JA, Lin Z, Vasta GR (2008) Transfection of the protozoan parasite Perkinsus marinus. Mol Biochem Parasitol 157:44–53
Gornik SG, Maegele I, Hambleton EA, Voss PA, Waller RF, Guse A (2022) Nuclear transformation of a dinoflagellate symbiont of corals. Front Mar Sci 9:1035413
Hoegh-Guldberg O (1999) Climate change, coral bleaching and the future of the world’s coral reefs. Mar Freshwater Res 50:839–866
Howells EJ, Abrego D, Liew YJ, Burt JA, Meyer E, Aranda M (2021) Enhancing the heat tolerance of reef-building corals to future warming. Sci Adv 7:eabg6070
Hughes TP, Anderson KD, Connolly SR et al (2018) Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359:80–83
Hughes TP, Kerry JT, Álvarez-Noriega M et al (2017) Global warming and recurrent mass bleaching of corals. Nature 543:373–377
Jinkerson RE, Russo JA, Newkirk CR, Kirk AL, Chi RJ, Martindale MQ, Grossman AR, Hatta M, Xiang T (2022) Cnidarian-Symbiodiniaceae symbiosis establishment is independent of photosynthesis. Curr Biol 32:2402-2415.e4
Kwok ACM, Chan WS, Wong JTY (2023) Dinoflagellate amphiesmal dynamics: Cell wall deposition with ecdysis and cellular growth. Mar Drugs 21:70
Levin RA, Suggett DJ, Nitschke MR, van Oppen MJH, Steinberg PD (2017a) Expanding the Symbiodinium (Dinophyceae, Suessiales) toolkit through protoplast technology. J Eukaryot Microbiol 64:588–597
Levin RA, Voolstra CR, Agrawal S, Steinberg PD, Suggett DJ, van Oppen MJH (2017b) Engineering strategies to decode and enhance the genomes of coral symbionts. Front Microbiol 8:1220
Lohuis MRT, Miller DJ (1998) Genetic transformation of dinoflagellates (Amphidinium and Symbiodinium): expression of GUS in microalgae using heterologous promoter constructs. Plant J 13:427–435
Marinov GK, Trevino AE, Xiang T, Kundaje A, Grossman AR, Greenleaf WJ (2021) Transcription-dependent domain-scale three-dimensional genome organization in the dinoflagellate Breviolum minutum. Nat Genet 53:613–617
Morrill LC, Loeblich AR (1983) Ultrastructure of the dinoflagellate amphiesma. Int Rev Cytol 82:151–180
Ortiz-Matamoros MF, Islas-Flores T, Voigt B, Menzel D, Baluška F, Villanueva MA (2015) Heterologous DNA uptake in cultured Symbiodinium spp. aided by Agrobacterium tumefaciens. PLoS One 10:e0132693
Paternostre MT, Roux M, Rigaud JL (1988) Mechanisms of membrane protein insertion into liposomes during reconstitution procedures involving the use of detergents. 1. Solubilization of large unilamellar liposomes (prepared by reverse-phase evaporation) by Triton X-100, octyl glucoside, and sodium cholate. Biochemistry 27:2668–2677
Potrykus I, Shillito RD (1986) Protoplasts: Isolation, culture, plant regeneration. Methods Enzymol 118:549–578
Qin S, Lin H, Jiang P (2012) Advances in genetic engineering of marine algae. Biotechnol Adv 30:1602–1613
Sakamoto H, Lin XX, Bai YD, Chen XF, Zhang ZZ, Honjo Y, Hikosaka K (2022) Development of a novel electroporation method for the oyster parasite Perkinsus marinus. Sci Rep 12:19996
Seddon AM, Curnow P, Booth PJ (2004) Membrane proteins, lipids and detergents: not just a soap opera. Biochim Biophys Acta 1666:105–117
Spector DL (1984) Dinoflagellates. Academic Press, Inc., Orlando
Starko S, Fifer JE, Claar DC, Davies SW, Cunning R, Baker AC, Baum JK (2023) Marine heatwaves threaten cryptic coral diversity and erode associations among coevolving partners. Sci Adv 9:eadf0954
van Oppen MJH, Gates RD, Blackall LL et al (2017) Shifting paradigms in restoration of the world’s coral reefs. Glob Chang Biol 23:3437–3448
Wakefield TS, Farmer MA, Kempf SC (2000) Revised description of the fine structure of in situ “zooxanthellae” genus Symbiodinium. Biol Bull 199:76–84
Xiang T, Hambleton EA, DeNofrio JC, Pringle JR, Grossman AR (2013) Isolation of clonal axenic strains of the symbiotic dinoflagellate Symbiodinium and their growth and host specificity. J Phycol 49:447–458
Acknowledgements
The authors thank the following for their contributions to this work: Prof. Eugene Nothnagel, UCR Department of Botany and Plant Sciences; Dr. Mary Hamer, UCR School of Medicine Cell Sorter & Flow Cytometry Facility; Dr. David Carter, UCR Microscopy & Imaging Core Facility; Dr. Rachel Behar, UCR Stem Cell Core Facility; and Mr. Joseph Russo, UCR Department of Microbiology.
Funding
UCR Delfino Agriculture Innovation Seed Fund, Department of Education Graduate Assistance in Areas of National Need (GAANN), and National Science Foundation Graduate Research Fellowship (NSF GRFP).
Author information
Authors and Affiliations
Contributions
All authors contributed to the conception and design of the study. Data collection and analyses were performed by P.I.P. The manuscript was drafted by P.I.P. and reviewed by M.L.D., J.N.-V., M.L.O.-C., T.X., R.E.J, and M.P.R.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pairs, P.I., Dundon, M.L., Narváez-Vásquez, J. et al. Cell wall digestion of the dinoflagellate Breviolum minutum. J Appl Phycol 36, 181–189 (2024). https://doi.org/10.1007/s10811-023-03140-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-023-03140-7