Abstract
Seaweed extracts are proven to increase productivity in many agricultural crops, but there is limited research on their use in avocado production. Therefore, we evaluated the effectiveness of a seaweed extract from Durvillaea potatorum and Ascophyllum nodosum on avocado yield, revenue and post-harvest fruit quality in a series of field experiments in Australia, and on seedling root growth in a pot experiment. The field experiments were conducted on commercial farms across three different locations in northern Queensland over four years and utilised avocado trees with different ages, cultivars (Hass and Shepard) and inoculum pressures from Phytophthora cinnamomi. Results showed that the application of the seaweed extract by fertigation significantly improved avocado yield (kg fruit per tree) by 38%, fruit firmness by 4% (skin) and 22% (flesh) and fruit skin colour by 1° (hue), and an upgraded visual ripeness score. The increases in yield were associated with greater number of fruits per tree (up to 42%) indicating the liquid seaweed extract improved fruit set and retention per tree. Regular soil application of the seaweed extract to young trees (cv. Hass) in pots increased the root fresh weight by 22%. Overall, the regular application of the seaweed extract to avocado trees was found to be practical and economically viable for improving fruit production and post-harvest quality in Australian orchards.
Similar content being viewed by others
Introduction
Avocado (Persea americana Mill.) is an economically important tree crop grown in tropical and subtropical regions of many parts of the world, including Australia. Avocados have an important function in the diet due to their organoleptic and nutritional components, including a high content of unsaturated fatty acids and dietary fibre. These components are associated with decreased risk of cardiovascular disease and weight control, among others (Dreher and Davenport 2013; Duarte et al. 2016; Peou et al. 2016). As the worldwide demand for avocado fruits increases, so does the need to increase productivity of existing and new plantations using sustainability and regenerative principles.
The productivity of avocado trees is affected by many biotic and abiotic factors. Crop yield is limited by irregular (alternate) bearing of the fruit across production years and by genetics because there are few elite cultivars and rootstocks (Wallace and Wallace 1993). Crop yield is also affected by fruit set and abscission, which are dependent on many physiological processes and environmental factors including temperature and humidity (Sedgley 1977). The improvement of fruit set rates is especially relevant for increasing avocado yield. This is because fruit set is inherently low (< 0.1%) in avocado despite the abundant production of flowers by the tree (Pedreschi et al. 2019). Crop yield is also affected by pests and diseases such as Phytophthora root rot (caused by Phytophthora cinnamomi), climate variability (e.g., drought and heatwaves), and soil conditions (e.g., salinity) (Pegg et al. 1982; Guest and Grant 1991; Bonomelli et al. 2018). Phytophthora root rot is amongst the most important factors that limit yields in most countries, including Australia. The pathogen infects the feeder roots causing disease that leads to loss of production due to reduced nutrient and water uptake and, without treatment, trees eventually die (Guest and Grant 1991; Reeksting et al. 2016).
It is well established that seaweed extracts can increase the yield and quality of many horticultural crops due to their biostimulant effects, including improvements in plant nutrient-use efficiency and tolerance of abiotic and biotic stresses (Khan et al. 2009; Mattner et al. 2013, 2018; Arioli et al. 2015; Brown and Saa 2015; Shukla et al. 2019; Hussain et al. 2021; Li et al. 2022). However, few studies on avocado have investigated whether seaweed extracts can increase yield (Morales-Payan and Candelas 2014; El-Shamma et al. 2017). In addition to yield issues, consumers are demanding avocado fruit produced under safe and environmentally sustainable production practices, and with good post-harvest quality. They demand fruit that is free of external and internal defects, high in organoleptic and nutritional attributes, and has an acceptable shelf-life (time to ripe).
In Australia there are no scientific publications reporting the effect of seaweed extracts on avocado productivity and post-harvest fruit quality. The aim of this study was to determine the effect of regular soil applications with a seaweed extract on avocado yield, post-harvest fruit quality and revenue in large-scale, field trials with Hass and Shepard cultivars across three locations and four seasons in Queensland, Australia. The effect of the seaweed extract on root growth was also investigated in a pot experiment with young trees (cv. Hass).
Materials & methods
Field trials
Seaweed extract treatment
The treatment evaluated in this study (Seasol, Seasol International, Australia) was an alkaline extract made from two cold-water seaweeds (Ascophyllum nodosum and Durvillaea potatorum) known to activate key growth, health, and molecular response pathways in plants, as identified by Arabidopsis transcriptomics research (Islam et al. 2020, 2021). Information about the seaweed extract composition has been previously published (Arioli et al. 2015). Except where otherwise stated, the seaweed extract was applied at 10 L ha−1 (diluted 1:400 concentration) and compared with untreated controls that received equivalent amount of irrigation water. The treatment application rate and concentration were chosen based on efficacy studies conducted in Australia (Mattner et al. 2013, 2018; Farnsworth and Arioli 2018; Arioli et al. 2020). The treatments were applied at regular (monthly) intervals from harvest to harvest (i.e., 12 treatments/year) using fertigation systems and micro-sprinklers beside each tree. The regular application strategy used in the field trials aimed at pre-conditioning avocado trees, by continuous activation of biostimulant effects, to stress events that limit yields.
Locations and soil assessment
Field trials were conducted over five growing seasons (2017–2021) at three commercial farms in northern Queensland, Australia (Table 1). Irrigation water, fertiliser and pesticides were applied equally, based on the growers’ standard practices at each site, to the two treatments.
Field site 1 (2017–18) was established in a 6-ha block of 12-year-old Shepard trees transplanted into a red-brown silty clay loam in 2004 (Table 1). The trees were planted using an 8 m × 6 m spacing (250 trees per hectare). The block was divided into two sections, approximately 60 m wide and 500 m long, with each section representing a single plot of each treatment.
Field site 2 (2018–21) was established in a 6-ha block of 3-year-old Shepard trees transplanted into a red-brown silty clay loam in 2016 (Table 1). The block had a 5% slope with ten rows of trees planted in a 10 m × 6 m spacing (300 trees per hectare). The grower used a heavy cover of straw mulch underneath the trees to increase organic matter and suppress weed growth. Treatments were evaluated over three seasons (2018–19; 2019–20; 2020–21) and the experiment was set up as a randomized complete block design with four blocks. At this site, the seaweed extract was applied every 2-weeks (5 L ha−1), which was equivalent to 10 L ha−1 per month.
Field site 3 (2018–19) was established in a 16-ha block of 6-year-old Hass trees transplanted into a red clay loam soil in 2012 (Table 1). The trees were planted using a 9 m × 12 m spacing (250 trees per hectare). The grower at this site used a heavy cover of straw mulch underneath the trees to suppress weed growth. The block was divided into two sections, each representing a single plot of each treatment. At this trial, 7.5 L ha−1 of the seaweed extract was applied every four weeks in the first six months, and 10 L ha−1 thereafter.
In 2021, soil across each site was analysed for background levels of P. cinnamomi to quantify the relative risk of Phytophthora root rot in avocado trees (Table 1). In this procedure, fifty soil cores were sampled from 0–15 cm in a W-pattern across the whole block of field sites 1 and 3. Soil was thoroughly mixed and a sub-sample of 450 g submitted to a commercial laboratory (South Australian Research and Development Institute, Waite Campus, Adelaide, South Australia), DNA extracted, and qPCR performed for P. cinnamomi using the general procedures described by Ophel-Keller et al. (2008). The efficiency and consistency of SARDI’s method has been confirmed in comparison with commercial extraction kits (Haling et al. 2011). For site 2, soil samples from each plot (two composite samples of 450 g from 0–15 cm) were collected and analysed for concentrations of DNA of P. cinnamomi as described previously.
Avocado yield
Harvest occurred between February and March at each site when fruit had reached physiological maturity. The timing of harvest (i.e., optimum fruit maturity) was determined based on industry dry matter concentration standards. Yield was assessed by picking, grading, and weighing all avocado fruit per tree. At field sites 1 and 3, fruit were harvested from 4 – 6 randomly selected trees along four rows in each treatment (i.e., 16 – 24 trees per treatment). At field site 2, fruit were harvested from 16 randomly selected trees in each of the four blocks of treatments (i.e., 64 trees per treatment). Fruit were graded in the packing shed as premium (blemish free), marketable (with minor blemishes) or unmarketable (moderate to severe blemishes or deformations or undersize).
Following flowering at field site 2 (2020/21), the number of fruit on each tree were counted at eight regular intervals from August to February. Assessments at each time interval were made on 2–5 trees per plot, depending on the total number of fruit present.
Partial budget analysis
A partial budget analysis (AU$) on the use of the seaweed extract on avocado production at site 2 in 2020/21 was determined using the method described by Szparaga et al. (2019). Fruit from all replicates for each treatment were pooled and pack-out yields determined by the farmer. Calculations were based on: (i) the increase in revenue from fruit from the use of the seaweed extract, and (ii) the costs to purchase and apply the product in Australia. The relevant wholesale avocado prices for the Shepard cultivar, region and year were sourced from the farmer and the avocado wholesale prices were averaged across the grades and sizes for the revenue calculations. The costs for the seaweed extract were based on frequency and rate of application. The only differential cost between treatments was for the seaweed extract concentrate (AU$40 per 10 L). Application of the seaweed extract occurred at the same time as normal fertigation practices, so did not incur additional costs in water, infrastructure, operation, depreciation, or labour.
Postharvest experiments
Avocado fruit (cv. Shepard) used in post-harvest experiments were collected from trees at Sites 1 and 2 over four seasons from 2016–2021 (Table 1). The fruit were immediately cooled, stored at 7 °C for up to 24 h, and then transported at 7 °C in single-layer cartons to the laboratory. Post-harvest experiments commenced immediately after fruit arrived, 4 to 6 days after commercial harvest. The fruit were not treated with post-harvest fungicides or ethylene gas to initiate ripening.
Experimental design of post-harvest experiments
The experiment was designed to determine the effect of the seaweed extract applied to trees in the field on fruit quality parameters (see below) during avocado storage and ripening. The design consisted of two cold storage treatments (none, or storage for 21 days at 7 °C and 90% RH), and two ripening scenarios (5 and 4 days at 18 °C and 70% RH, respectively). Fruit from the control and seaweed extract treatments from the field were randomly assigned to four experimental units per storage by ripening combination (i.e., four replicates per treatment combination). Individual experimental units consisted of five avocado fruit which were stored in plastic-lined, single-layer cartons.
Measurement of fruit quality parameters
Fruit firmness was measured on both cheeks of each fruit at its widest point with a hand-held digital tester (Durofel DFT 100, Agrosta, France) using the Shore A hardness 0 to 100 scale, where 0 = extra soft, 20 = soft, 40 = medium soft, 70 = medium hard and > 90 = hard (White et al. 2003). Measurements were taken with the skin intact and on the flesh of the fruit with the skin removed. Areas of the fruit with suspected bruises (soft spots) were avoided during the measurements. The firmness tester was calibrated to zero and 100 hardness units prior to measurements.
Skin color of the surface of the avocado was measured at four equidistant points along the widest diameter of each fruit with a hand-held tristimulus reflectance colorimeter (model CM-2600d, Minolta Corp., USA). Color was recorded using the CIE L*a*b* uniform color space (CIE Laboratories), where L* indicates lightness, a* indicates chromaticity on a green (-) to red ( +) axis, and b* chromaticity on a blue (-) to yellow ( +) axis (Hofman and Jobin-Décor 1999). Numerical values of a* and b* for each fruit were averaged and then the hue angle calculated using H° = arctan (b*/a*).
A five-point rating scale was used to describe visual ripeness of each fruit where 1 = unripe (emerald green and shiny); 2 = onset ripe (forest green and < 20% dark); 3 = ripe (black on green and 20 to 30% dark); 4 = eating ripe (green on black and 60 to 70% dark), and 5 = over-ripe (mostly black and > 70% dark) (White et al. 2003).
Prior to cold storage and/ or ripening, five fruit were randomly selected from each replicate within a treatment and the cheek of each side of a fruit was sliced off, skin peeled off, and the flesh of each cheek trimmed down to approximately 15–30 g weight. Flesh pieces were then placed in individual paper bags, weighed, and dried to constant weight at 65 °C. Dried avocado cheeks were then weighed again and the dry matter concentration calculated as a percentage of the fresh sample weight (i.e., (dry weight/fresh weight) × 100 = % Dry matter).
Pot experiment
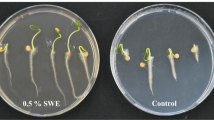
A pot experiment conducted at Bayswater, Victoria, in 2017/18 investigated the effect of the seaweed extract on root growth of 12-month-old avocado trees (grafted Hass on Hass), using the method described by Zodapi (2001). The avocado trees were potted in 400-mm diameter plastic pots containing premium potting mix with fertilizer (Debco®, Australia). Treatments included the seaweed extract (as described above) and water as the untreated control. Five litres of the seaweed extract (1:400 dilution) were applied to treated pots every two weeks (12 applications in total), which watered the potting mix to field capacity. Five litres of water were applied to the control pots. All plants were grown in an open area covered with netting, fertilized every six weeks (125 g fertilizer granules per plant (Powerfeed®, Seasol International, Australia)), and irrigated to field capacity for the duration of the trial (6 months from spring to summer). The experiment was repeated and conducted as a randomized complete block design, with four blocks. The first experiment consisted of 12 and 13 pots for the water control and seaweed extract treatment respectively, and the second of 3 pots (each).
The effect of the treatments on root growth was determined by measuring the fresh weights of roots as described by Huang et al. (2017) and before their root growth was restricted by the size of the pot. Root growth was monitored using a clear plastic window (A4-size, transparent plastic sheet) placed on one side of one pot per treatment. When the first roots reached 80% of the pot depth in the two pots with windows, the roots of three avocado trees per treatment (without the plastic windows) were dug up. The roots were then carefully soaked in a tub containing tap water overnight and rinsed gently the next morning to remove all potting mix soil from roots. Roots were thoroughly blotted dry on adsorbent paper and fresh weights determined by weighting.
Statistical analysis
The yield data were analysed using linear mixed models which were appropriate for incorporating the structure of the data arising from the layout of the field trials. Four levels of variation were included in the analysis: between sites, between blocks within sites, between plots within blocks, and (for some plots) between measurements within plots. The variation between plots was then used to compare the two treatments (seaweed extract versus untreated control). If the variation between sites or blocks within sites was estimated to be minimal, then the effect of that factor was not incorporated in the analysis, and fewer levels of variation were used in the statistical model. Fruit number data were also analysed by linear mixed models, with rows nested within plots and samples nested within rows in the analysis. If a plot of residuals versus fitted values showed increasing variation with the mean, then the yield and fruit number data were loge transformed before analysis, with a small increment added if zeros were present in the data. In these cases, statistical inference was performed on the transformed data, but the treatment means were back-transformed to the original scale for easy comparison.
For post-harvest data, linear mixed models were fitted which incorporated the complex random effects structure arising from the combination of field trials and post-harvest experiments. If variation at a particular level of the structure was estimated to be minimal or the algorithm did not converge, the random effects were simplified to obtain fewer levels of variation. The fixed effects included the main effects of treatment, year and storage assessment, and the interaction between treatment and storage assessment (the treatment by year interaction was not significant for any outcome). Plots of residuals vs fitted values were constructed to assess homogeneity of variance, and were found to adequately support this assumption. All statistical analyses were conducted using GenStat (VSN International, UK).
Results
Field trials
Overall results
The potential for root rot risk was confirmed by the detection of background levels of P. cinnamomi at the trial sites (Table 1). In commercial-scale field trials, the seaweed extract consistently increased the yield of avocado grown under contrasting conditions and environments. On average, the seaweed extract treatment significantly (P = 0.038) increased avocado yield by 38%, compared with untreated trees, across the sites and years (i.e., the year × treatment interaction was not significant) (Table 2).
Results from site 2 (2020/21)
In 2020–21, trees treated with seaweed extract produced a significantly (P = 0.015) greater number of fruit (42% more overall) than those in the untreated control (Table 3). Differences in fruit numbers between trees in the seaweed extract and the control treatments were not significantly different at any individual monthly assessment (except in February). The treatment by time of assessment interaction was not significant, indicating that the effect of the seaweed extract was consistent across assessments dates. Treatment with the seaweed extract did not significantly affect the concentration of P. cinnamomi in soil after harvest. Average concentrations of P. cinnamomi in the untreated control and in the seaweed extract treatment were 53 pg DNA g soil−1 (Table 1). The use of the seaweed extract increased pack-out yield of avocado by 26%. This resulted in an increase in revenue from fruit in the seaweed extract treatment of 24% and an increased partial profit by AU$8,632 ha−1 (Table 4).
Postharvest experiments
The skin and flesh of avocado fruit from the seaweed extract treatment was significantly (P = 0.014 and P = 0.008) firmer by 4% and 22%, respectively, than fruit from the untreated control (Table 5). There was evidence that fruit collected from seaweed extract-treated trees had significantly (P = 0.050) higher hue angle values for color, by 1°, than those from the untreated control following ripening immediately after harvest, and directly out of cold storage. (Table 5). This indicates that fruit from trees treated with the seaweed extract treatment had less skin darkening (i.e., greener skin during ripening) than those from the control, which is a favorable trait. Fruit from seaweed extract-treated trees had significantly (P < 0.047) favorable (reduced) ripeness scores compared with the untreated control. (Table 5). Figure 1 shows the interactions between storage and ripening treatments on avocado fruit. Results indicated that all avocado fruit met commercial requirements for dry matter concentration. Average dry matter concentration of fruit was 26.6% for both treatments.
Effect of fertigation with a seaweed extract (grey columns) on skin firmness (A), flesh firmness (B), skin colour (C), and fruit ripeness (D) of avocado (cv. Shepard) after postharvest storage and ripening, compared with the untreated control (white columns). Results are averaged across two locations (Site 1 and 2) and four years (2017, 2018, 2019 and 2021)
Pot experiment
In the pot experiment, the seaweed treatment significantly increased the fresh weight (P = 0.022) of avocado roots by 21.8%, compared with the untreated control (Table 6).
Discussion
This series of field experiments is currently the most comprehensive evaluation of the effect of a seaweed biostimulant on commercial production of avocado in Australia. Results showed that the integrated use of a seaweed extract made from Ascophyllum nodosum and Durvillaea potatorum with other conventional production practices increased avocado fruit yield by 38% and postharvest quality (i.e., firmness, color and shelf-life) in an economically beneficial and sustainable manner. Results were consistent across two cultivars of avocado, contrasting tree ages, four years of production, and different locations within northern Queensland, Australia. The effect could be generated within a single season as well as over time, and in soils with different levels of inoculum pressure from P. cinnamomi. Yield increases by the seaweed extract were associated with greater fruit set in the field and increased root growth in a complementary pot experiment.
The findings of this study are similar to those in the limited number of papers in the scientific literature that have evaluated the effect of seaweed extracts on avocado yield. Morales-Payan and Candelas (2014) found that six foliar applications of a seaweed extract from A. nodosum at a rate of 4 L ha−1 increased the number of avocado fruit (cv. Butler) retained per tree when used in combination with a standard fertilizer program. Similarly, El-Shamma et al. (2017) showed that soil applications of a seaweed extract from Sargassum sp., Laminaria sp., and A. nodosum (Alga 600™) in combination with a microbial biostimulant increased the fruit yield of avocado (cv. Fuerte) by an average of 30% compared with the microbial biostimulant alone. More broadly, the avocado productivity improvements we found are consistent with other crop studies (broccoli, strawberry, tomato, wine grape, sugarcane) using the same seaweed extract (Mattner et al. 2013, 2018; Arioli et al. 2015, 2021a, b; Hussain et al. 2021).
One of the main limitations to avocado productivity worldwide is the considerable rate of abscission of flowers and small fruit during the first two months after the commencement of anthesis. As a result, the natural fruit set is usually low (< 0.15%) compared with the flowers initially produced by the tree (Sedgley 1977; Garner and Lovatt 2008; Pedreschi et al. 2019). In the current experiments, the seaweed extract treatment increased the number of fruit per tree by 21–75% during key stages of development from late winter to spring when fruit drop occurs. This result suggests that one of the physiological mechanisms by which the seaweed extract increased avocado yield was by reducing flower and fruit abscission. This result is consistent with previous studies in avocado with seaweed extracts (Morales-Payan and Candelas 2014; El-Shamma et al. 2017). Some of the factors involved in the abscission of flowers and fruit during development include extreme temperatures (Sedgley 1977), the productive alternation (Garner and Lovatt 2008), and nutritional deficiencies (Lahav and Zamet 1999). Therefore, the increased fruit set by trees treated with the seaweed extract in the current experiments may relate to increased tolerance of these specific abiotic factors.
The primary roots of avocado are superficial and lack absorbent hairs, so their shallow white feeder roots are used to capture nutrients from the soil (Pegg et al. 1982). A pot experiment in the current study showed application of the seaweed extract increased fresh weight of avocado roots by 21.8%. Our results are consistent with other publications where seaweed extracts have improved lateral formation (Artzamon and Staden 1994), increased initiation (Alam et al. 2013), extended elongation (Rayorath et al. 2008; Arioli et al. 2015; Islam et al. 2020) and increased biomass (El-Miniawy et al. 2014; Mattner et al. 2018) of roots of other crops. The shallow root system of avocado makes the crop particularly susceptible to abiotic and biotic stresses. The finding that the seaweed extract increased root growth is particularly relevant to avocado because it is one mechanism that may confer increased tolerance for stress and improved yields.
Results from the current experiments demonstrated that repeated applications with the seaweed extract improved the post-harvest quality of avocados in terms of enhancing the useful life (shelf-life). Firmness (skin and flesh) and color (hue and visual ripening score) are both post-harvest indicators of avocado fruit shelf-life in the market. Use of the seaweed extract consistently increased post-harvest firmness and color of avocado fruit (cv Shepard) across different seasons, locations, and storage and ripening conditions commonly used in the Australian supply chain and market. Several factors can influence the post-harvest firmness of avocado, including the concentration of calcium in the fruit (Witney et al. 1990; Rivera et al. 2017), which is in turn influenced by water uptake (Witney et al. 1990). Furthermore, temperature of the season, water availability, and the nitrogen content of the fruit are important variables that influence the change in skin color during the storage of ‘Hass’ avocado (Cox et al. 2004; Rivera et al. 2017). Therefore, it is possible that the use of the seaweed extract improved nutrient or water uptake by trees in the current experiments to effect improvements in fruit quality. This hypothesis is supported by results of previous studies where application of seaweed extracts has increased calcium (e.g., Crouch et al. 1990), nitrogen (e.g., Rathore et al. 2009; Jannin et al. 2013) and water (e.g., Shukla et al. 2018) uptake/content in plants. In contrast to parameters of effective fruit life, the use of the seaweed extract in the current experiments showed little evidence of influencing dry matter percentage of avocado fruit.
Our results strongly support the biostimulant effect of the seaweed extract to increase avocado yield, profitability and fruit shelf-life. However, the exact mode of action by which the seaweed extract achieved the benefits needs further investigation. It is unlikely that the improvements in avocado yields and changes in post-harvest fruit quality were due to a fertilizer effect of the seaweed extract because its nutrient composition is low (Wite et al. 2015) and the current trials were conducted using a synthetic fertilizer program for crop nutrition. Research has also determined that the nutrient content alone in the seaweed extract used in this study had no effect stimulating plant growth (Yusuf et al. 2012).
Numerous studies have established the effects of different seaweed extracts in modulation of plant gene expression and the status of metabolites and reactive oxygen species (ROS) signalling molecules (Jannin et al. 2013; Goni et al. 2016; Frioni et al. 2019; Islam et al. 2020, 2021; Omidbakhshfard et al. 2020; Staykov et al. 2021). Furthermore, transcriptomics research supports the notion that seaweed extracts may act as a plant priming stimulant that results in a faster and/or stronger induction of plant defenses (Islam et al. 2020, 2021; Rasul et al. 2021). Plant-priming occurs in a wide range of plant species and is often associated with enhanced abiotic and biotic stress tolerance (Martinez-Medina et al. 2016).
Arabidopsis transcriptomics, cellular biology and phenotyping research has shown the same seaweed extract-based biostimulant used in this study had the ability to activate key plant growth, health and other molecular response pathways (Islam et al. 2020, 2021). The studies showed that the extent of colonization by P. cinnamomi in infected roots of A. thaliana was suppressed by pre-treatment with the seaweed extract. This response was associated with the up-regulation of specific defense-associated pathways such as systematic acquired resistance (SAR) (and others regulated by salicylic acid, jasmonic acid and ethylene) and genes associated with plant resistance (for example PR1, MLO, and others). SAR is a systemic mechanism in plants and is known to increase plant tolerance to different types of biotic and abiotic stresses (Klessig et al. 2018; Wang et al. 2018). In the current experiments, the use of the seaweed extract did not reduce concentrations of P. cinnamomi in soil, but did increase avocado yield across sites with varying inoculum pressure from the pathogen. Therefore, it is possible that the stimulation of SAR was an important factor that contributed to the yield response in avocado at these sites.
The adoption of more sustainable farming practices can be challenging for growers. Research has found growers are unlikely to implement sustainable farm practices unless they provide advantages such as economic, environmental, business, safety or are required by government policy (Kallas et al. 2010; Cullen et al. 2013; Forbes et al. 2013). An increasing number of publications are finding that the use of seaweed extracts is economically viable when incorporated into conventional farming (Pal et al. 2015; Singh et al. 2015; Mattner et al. 2018; Arioli et al. 2021a, b).
The results from the current trials indicate that the economics of using the seaweed extract in avocado production can be favourable for producers in Australia. A partial revenue analysis using data from the most recent field trial (2020–21) found the use of seaweed extract increased the avocado marketable yield (fruit trays) per tree by 21% and this resulted in an estimated 24% increase in grower’s returns or AU$ 9,111 ha−1 at the trial site. Most avocado growers have the infrastructure to apply the seaweed extract through their irrigation system and as additives to their spray programs, and therefore there would be no additional costs from the treatment. The intangible economic benefit of using the extract not included in the analysis was the increased shelf-life of fruit.
In addition to the economic benefits, seaweed extracts offer other practical benefits. Seaweed extracts are complimentary to crop-protection solutions, are inherently biodegradable and safe to apply and their effectiveness is proving to be useful to many crops, especially under conditions of stresses due to seasonal volatility and climate anomalies (Shukla et al. 2019; Li et al. 2022). Seaweed extracts have the practical attribute of being applied by different methods such as soil irrigation, foliar sprays, drenching and soaking. Consequently, seaweed extracts are an important management option in the transition to sustainable avocado production.
Conclusion
This study found that regular soil applications with a seaweed extract made from two seaweeds (Durvillaea potatorum and Ascophyllum nodosum) increased avocado productivity (avocado yield, fruit number, root growth), production revenue and post-harvest fruit quality (firmness and colour). These post-harvest characteristics are important for enhancing shelf-life and consumer acceptance of fruit. A deeper understanding of the mechanisms by which seaweed extracts promote enhanced root growth, tree productivity and post-harvest fruit quality may offer new approaches for sustainable avocado production.
Data availability
Available data is provided in the publication.
References
Alam MZ, Brown G, Norrie J, Hodges DM (2013) Effect of Ascophyllum extract application on plant growth, fruit yield and soil microbial communities of strawberry. Can J Plant Sci 93:23–36
Arioli T, Hepworth G, Farnsworth B (2020) Effect of seaweed-extract application on sugarcane production. Proc Aust Soc Sugar Cane Technol 42:393–396
Arioli T, Hepworth G, Farnsworth B, Kasinadhuni N, Noune C, Mattner S (2021a) Effect of seaweed-extract application on sugarcane yield in Australia. Proc Aust Soc Sugar Cane Technol 42:637–644
Arioli T, Mattner S, Hepworth G, McClintock D, McClintock R (2021b) Effect of seaweed extract application on wine grape yield in Australia. J Appl Phycol 33:1883–1891
Arioli T, Mattner S, Winberg P (2015) Applications of seaweed extracts in Australian agriculture: past present and future. J Appl Phycol 27:2007–2015
Artzamon N, Staden J (1994) The effect of seaweed concentrate on the growth of Pinus pinea seedlings. New Forest 8:279–288
Bonomelli C, Celis V, Lombardi G, Mártiz J (2018) Salt stress effects on avocado (Persea americana Mill.) plants with and without seaweed extract (Ascophyllum nodosum) application. Agronomy 8(5):64
Brown P, Saa S (2015) Biostimulants in agriculture. Front Plant Sci 6:671
Cox KA, McGhie TK, White A, Woolf AB (2004) Skin colour and pigment changes during ripening of Hass avocado fruit. Postharvest Biol Technol 31:287–294
Crouch IJ, Beckett RP, van Staden J (1990) Effect of seaweed concentrate on the growth and mineral nutrition of nutrient-stressed lettuce. J Appl Phycol 2:269–272
Cullen R, Forbes SL, Grout R (2013) Non-adoption of environmental innovations in wine growing. NZ J Crop Hort Sci 41:41–48
Dreher M, Davenport A (2013) Hass avocado composition and potential health effects. Crit Rev Food Sci Nutr 53:738–750
Duarte F, Chaves A, Borges D, Mendonça B (2016) Avocado characteristics, health benefits and uses. Ciênc Rural 46:747–754
El-Miniawy SM, Ragab ME, Youssef SM, Metwally AA (2014) Influence of foliar spraying of seaweed extract on growth, yield and quality of strawberry plants. J Appl Sci Res 10:88–94
El-Shamma S, Helal M, Maksoud M, Khalil F, Mansour A (2017) Effect of some bio-stimulants on nutritional status, yield and fruit quality of avocados. Middle East J Age Res 6:692–699
Farnsworth W, Arioli A (2018) Assessment of the yield of sugarcane following treatment with liquid seaweed extract (SEASOL®). Acta Hortic 1205:785–788
Forbes SL, Cullen R, Grout R (2013) Adoption of environmental innovations: analysis from the Waipara wine industry. Wine Econ Policy 2:11–18
Frioni T, Tombesi S, Quaglia M, Calderini O, Moretti C, Poni S, Gatti M, Moncalvo A, Sabbatini P, Berrios J, Palliotti A (2019) Metabolic and transcriptional changes associated with the use of Ascophyllum nodosum extracts as tools to improve the quality of wine grapes (Vitis vinifera cv. Sangiovese) and their tolerance to biotic stress. J Sci Food Agric 99:6350–6363
Garner LC, Lovatt CJ (2008) The relationship between flower and fruit abscission and alternate bearing of ‘Hass’ avocado. J Am Soc Hort Sci 133:3–10
Goni O, Fort A, Quille P, Mckeown P, Spillane C, O’Connell S (2016) Comparative transcriptome analysis of two Ascophyllum nodosum extract Biostimulants: same seaweed but different. J Agric Food Chem 64:2980–2989
Guest I, Grant B (1991) The complex action of phosphonates as antifungal agents. Biol Rev 66:159–187
Haling RE, Simpson RJ, McKay AC, Haryley D, Lambers H, Ophel-Keller K, Wiebkin S, Riley IT, Richardson AE (2011) Direct measurement of roots in soil for single and mixed species using a quantitative DNA-based method. Plant Soil 348:123–137
Hofman P, Jobin-Décor M (1999) Effect of fruit sampling and handling procedures on the percentage dry matter, fruit mass, ripening and skin colour of ‘Hass’ avocado. J Hort Sci Biotechnol 74:277–282
Huang P, de-Bashan L, Crocker T, Kloepper W, Bashan Y (2017) Evidence that fresh weight measurement is imprecise for reporting the effect of plant growth-promoting (rhizo) bacteria on growth promotion of crop plants. Biol Fertil Soils 53:199–208
Hussain H, Kasinadhuni N, Arioli T (2021) The effect of seaweed extract on tomato plant growth, productivity and soil. J Appl Phycol 33:1305–1314
Islam MT, Arioli T, Cahill DM (2021) Seaweed Extract-Stimulated Priming in Arabidopsis thaliana and Solanum lycopersicum. Plants 10:2476
Islam M, Gan H, Ziemann M, Hussain H, Arioli T, Cahill D (2020) Phaeophyceaean (brown algal) extracts activate plant defense systems in Arabidopsis thaliana challenged with Phytophthora cinnamomi. Front Plant Sci 11:852
Jannin L, Arkoun M, Etienne P, Liane P, Goux D, Garnica M, Fuentes M, San Franscisco S, Baigorri R, Cruz F, Houdusse F, Garcia-Mina J-M, Yvin J-C, Ourry A (2013) Brassica napus growth is promoted by Ascophyllum nodosum (L). Le Jol. seaweed extract: microarray analysis and physiological characterization of N, C, and S metabolisms. J Plant Growth Regul 32:31–52
Kallas Z, Serra T, Gil GM (2010) Farmers’ objectives as determinants of organic farming adoption: the case of Catalonian vineyard production. Agric Econ 41:409–423
Khan W, Rayirath U, Subramanian S, Jithesh M, Rayorath P, Hodges D, Critchley A, Norrie J, Prithiviraj B (2009) Seaweed extracts as biostimulants of plant growth and development. J Plant Growth Regul 28:386–399
Klessig D, Choi H, Dempsey D’M (2018) Systemic acquired resistance and salicylic acid: past, present, and future. MPMI 31:871-888
Lahav E, Zamet DN (1999) Flower, fruitlets and fruit drop in avocado trees. Rev Chapingo Ser Hortic 5:95–100
Li J, Van Gerrewey T, Geelen D (2022) A meta-analysis of biostimulant yield effectiveness in field trials. Front Plant Sci 13:836702
Martinez-Medina A, Flors V, Heil M, Mauch-Mani B, Pieterse C, Pozo M, Ton J, Dam N, Conrath U (2016) Recognizing plant defense priming. Trends Plant Sci 21:818–822
Mattner S, Milinkovic M, Arioli T (2018) Increased growth response of strawberry roots to a commercial extract from Durvillaea potatorum and Ascophyllum nodosum. J Appl Phycol 30:2943–2951
Mattner S, Wite D, Riches D, Porter I, Arioli T (2013) The effect of kelp extract on seedling establishment of broccoli on contrasting soil types in southern Victoria, Australia. Biol Agric Hortic 29:258–270
Morales-Payan P, Candelas D (2014) Increasing organic avocado fruit yield using a Ascophyllum nodosum biostimulant and fertilization. Acta Hortic 1042:121–124
Omidbakhshfard A, Sujeeth N, Gupta S, Omranian N, Guinan J, Brotman Y, Nikoloski Z, Fernie A, Mueller-Roeber B, Gechev T (2020) A biostimulant obtained from the seaweed Ascophyllum nodosum protects Arabidopsis thaliana from severe oxidative stress. Int J Mol Sci 21:474
Ophel-Keller K, McKay A, Hartley D, Herdnina H, Curran J (2008) Development of a routine DNA-based testing service for soilborne diseases in Australia. Australas Plant Pathol 37:243–253
Pal A, Dwivedi K, Maurya K, Kanwar P (2015) Effect of seaweed saps on growth, yield, nutrient uptake and economic improvement of maize (sweet corn). J Appl Nat Sci 7:970–975
Pedreschi R, Uarrota V, Fuentealba C, Alvaro J, Olmedo P, Defilippi B, Meneses C, Campos-Vargas R (2019) Primary metabolism in avocado fruit. Front Plant Sci 10:795
Pegg G, Forsberg J, Whiley W (1982) Avocado root rot. Qld Agric J 108:162–168
Peou S, Milliard-Hasting B, Shah S (2016) Impact of avocado-enriched diets on plasma lipoproteins: A meta-analysis. J Clin Lipidol 10:161–171
Rasul F, Gupta S, Olas J, Gerchev T, Sujeeth N, Mueller-Roeber B (2021) Priming with a seaweed extract strongly improves drought tolerance in Arabidopsis. Int J Mol Sci 22:1469
Rathore SS, Chaudhary DR, Boricha GN, Ghosh A, Bhatt BP, Zodape ST, Patolia JS (2009) Effect of seaweed extract on the growth, yield and nutrient uptake of soybean (Glycine max) under rainfed conditions. S Afr J Bot 75:351–355
Rayorath P, Jithesh M, Farid A, Khan W, Palanisamy R, Hankins S, Critchley A, Prithiviraj B (2008) Rapid bioassays to evaluate the plant growth promoting activity of Ascophyllum nodosum (L). Le Jol. Using a model plant Arabidopsis thaliana (L). Heynh. J Appl Phycol 20:423–429
Reeksting J, Oliver A, van den Berg N (2016) Transcriptome responses of an un-grafted Phytophthora root rot tolerant avocado (Persea americana) rootstock to flooding and Phytophthora cinnamomi. BMC Plant Biol 16:205
Rivera A, Ferreyra R, Robledo P, Sellés G, Arpaia L, Saavedra J, Defilippi G (2017) Identification of preharvest factors determining postharvest ripening behaviors in “Hass” avocado under long term storage. Sci Hortic 216:29–37
Sedgley M (1977) The effect of temperature on floral behaviour, pollen tube growth and fruit set in the avocado. J Hort Sci 52:135–141
Shukla PS, Shotton K, Norman E, Neily W, Critchley AT, Prithiviraj B (2018) Seaweed extract improve drought tolerance of soybean by regulating stress-response genes. AoB PLANTS 10: plx051
Shukla P, Mantin E, Adil M, Bajpai S, Critchley A, Prithiviraj B (2019) Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front Plant Sci 10:665
Singh SK, Thakur R, Singh MK, Singh CS, Pal SK (2015) Effect of fertilizer level and seaweed sap on productivity and profitability of rice (Oryza sativa). Indian J Agron 60:69–74
Staykov N, Angelov M, Petrov V, Minkov P, Kanojia A, Guinan K, Alseekh S, Fernie A, Sujeeth N, Gechev T (2021) An Ascophyllum nodosum-derived biostimulant protects model and crop plant from oxidative stress. Metabolites 11:24
Szparaga A, Kuboń M, Kocira S, Czerwińska E, Pawłowska A, Hara P, Kobus Z, Kwaśniewski D (2019) Towards sustainable agriculture – agronomic and economic effects of biostimulant use in common bean cultivation. Sustainability 11:4575
Wallace A, Wallace GA (1993) Limiting factors, high yields, and law of the maximum. Hortic Rev 15:409–448
Wang Y, Schuck S, Wu J, Yang P, Doring A-C, Zeier J, Tsuda K (2018) A MPK3/6-WRK33-ALD1-Pipecolic acid regulatory loop contributes to systemic acquired resistance. Plant Cell 30:2480–2494
White A, Hofman P, Lu Arpaia M, Woolf A (2003) The international avocado quality manual, Ver. 1.3. Hort Research, New Zealand
Wite D, Mattner S, Porter I, Arioli T (2015) The suppressive effect of a commercial extract from Durvillaea potatorum and Ascophyllum nodosum on infection of broccoli by Plasmodiophora brassicae. J Appl Phycol 27:2157–2161
Witney W, Hofman J, Wolstenholme N (1990) Effect of cultivar, tree vigour and fruit position on calcium accumulation in avocado fruits. Sci Hort 44:269–278
Yusuf R, Kristiansen P, Warwick N (2012) Potential effect of plant growth regulators on two seaweed products. Acta Horticult 958:133–138
Zodapi T (2001) Seaweed as a biofertilizer. J Sci Indust Res 60:378–382
Acknowledgements
We thank Hashmath Hussain and Jeffery Lang for their excellent technical work. The authors greatly acknowledge the growers who kindly assisted so the trials could be conducted scientifically at Australian Avocado orchards.
Funding
Research funding was provided by Seasol International Pty Ltd (Australia).
Author information
Authors and Affiliations
Contributions
All co-authors contributed to the final version of the work and approved the manuscript for publication. TA: project leader, interpretation of data, and writing the manuscript; OV performed all fruit quality research and interpretation of fruit quality data and writing the manuscript; GH: expert statistical analysis and writing the manuscript; BF: performed all the field trial research; SWM: interpretation of data and writing the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest/Competing interests
Seasol International (SI) is the manufacturer of the seaweed extract in Australia. TA is an employee of SI and an Adjunct Associate Professor at Deakin University. All other authors are not employees of SI. The authors declare that the research was conducted in the absence of any financial relationship that could be construed as a potential conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Arioli, T., Villalta, O.N., Hepworth, G. et al. Effect of seaweed extract on avocado root growth, yield and post-harvest quality in far north Queensland, Australia. J Appl Phycol (2023). https://doi.org/10.1007/s10811-023-02933-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10811-023-02933-0