Abstract
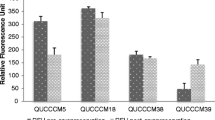
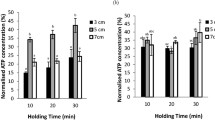
Marine microorganisms are gaining increased global importance owing to their richness in various bioactive compounds. The thraustochytrid Aurantiochytrium limacinum produces high amounts of the omega-3 fatty acid, DHA, and thus is of biotechnological importance. Aurantiochytrium limacinum cultures are usually maintained by continuous sub-culturing or plating, which is labour and resource-intensive. Having a cryopreservation technique will be useful for the preservation of A. limacinum cultures. Hence, we explored and developed a cryopreservation technique to preserve A. limacinum. Cryopreservation was done at two different temperatures by using a mechanical freezer (− 80 °C) and liquid nitrogen (− 196 °C). In the mechanical freezer, the cells were preserved in glycerol, while in liquid nitrogen four widely used cryoprotectants namely ethylene glycol, glycerol, methanol and dimethyl sulfoxide were used. The cells stored in a mechanical freezer had a relatively constant growth rate up to 12 months of storage. For liquid nitrogen storage, glycerol, methanol and dimethyl sulfoxide showed promising cryoprotectant activity with viability as high as 80% even after 12 months of storage. However, ethylene glycol did not exhibit any cryoprotective effect evidenced by marginal viability. Post-thaw assessment is crucial for judging the success of cryopreservation protocol and therefore, the study also looked into the functionality of cells on revival. On reviving no significant difference in the fatty acid profile and cell morphology among treatments was observed. The cryopreservation technique thus developed for preserving A. limacinum will help save human efforts and time and also conserve the organism’s genetic integrity.








Similar content being viewed by others
References
Abreu L, Borges L, Marangoni J, Abreu PC (2012) Cryopreservation of some useful microalgae species for biotechnological exploitation. J Appl Phycol 24:1579–1588
Barkia I, Saari N, Manning SR (2019) Microalgae for high-value products towards human health and nutrition. Mar Drugs 17:1–29
Beaty MH, Parker BC (1992) Cryopreservation of eukaryotic algae. Virginia J Sci 43:404–410
Brand JJ, Diller KR (2004) Application and theory of algal cryopreservation. Nova Hedwigia 79:175–189
Chen G, Fan KW, Lu FP, Li Q, Aki T, Chen F, Jiang Y (2010) Optimization of nitrogen source for enhanced production of squalene from thraustochytrid Aurantiochytrium sp. New Biotechnol 27:382–389
Chi Z, Liu Y, Frear C, Chen S (2009) Study of a two-stage growth of DHA-producing marine algae Schizochytrium limacinum SR21 with shifting dissolved oxygen level. Appl Microbiol Biotechnol 81:1141–1148
Cox SL, Hulston D, Maas EW (2009) Cryopreservation of marine thraustochytrids (Labyrinthulomycetes). Cryobiology 59:363–365
Crutchfield M, Diller KR, Brand JJ (1999) Cryopreservation of Chlamydomonas reinhardtii (Chlorophyta). Eur J Phycol 34:43–52
Day JG, Brand JJ (2005) Cryopreservation methods for maintaining microalgal cultures. In: Andersen RA (ed) Algal culturing techniques. Elsevier, Amsterdam, pp 165–187
Dellero Y, Cagnac O, Rose S, Seddiki K, Cussac M, Morabito C, Lupette J, Aiese Cigliano R, Sanseverino W, Kuntz M, Jouhet J, Maréchal E, Rébeillé F, Amato A (2018) Proposal of a new thraustochytrid genus Hondaea gen. nov. and comparison of its lipid dynamics with the closely related pseudo-cryptic genus Aurantiochytrium. Algal Res 35:125–141
Gwo JC, Chiu JY, Chou CC, Cheng HY (2005) Cryopreservation of a marine microalga, Nannochloropsis oculata (Eustigmatophyceae). Cryobiology 50:338–343
Hara A, Radin N (1978) Lipid extraction of tissues with a low toxicity solvents. Anal Biochem 90:420–426
Huang TY, Lu WC, Chu IM (2012) A fermentation strategy for producing docosahexaenoic acid in Aurantiochytrium limacinum SR21 and increasing C22:6 proportions in total fatty acid. Bioresour Technol 123:8–14
Kapoore RV, Huete-Ortega M, Day JG, Okurowska K, Slocombe SP, Stanley MS, Vaidyanathan S (2019) Effects of cryopreservation on viability and functional stability of an industrially relevant alga. Sci Rep 9:2093
Li J, Liu R, Chang G, Li X, Chang M, Liu Y, Jin Q, Wang X (2015) A strategy for the highly efficient production of docosahexaenoic acid by Aurantiochytrium limacinum SR21 using glucose and glycerol as the mixed carbon sources. Bioresour Technol 177:51–57
Morita E, Kumon Y, Nakahara T, Kagiwada S, Noguchi T (2006) Docosahexaenoic acid production and lipid-body formation in Schizochytrium limacinum SR21. Mar Biotechnol 8:319–327
Nakanishi K, Deuchi K, Kuwano K (2012) Cryopreservation of four valuable strains of microalgae, including viability and characteristics during 15 years of cryostorage. J Appl Phycol 24:1381–1385
Nakazawa A, Kokubun Y, Matsuura H, Yonezawa N, Kose R, Yoshida M, Tanabe Y, Kusuda E, Van Thang D, Ueda M, Honda D, Mahakhant A, Kaya K, Watanabe MM (2014) TLC screening of thraustochytrid strains for squalene production. J Appl Phycol 26:29–41
Park H, Kwak M, Seo JW, Ju JH, Heo SY, Park SM, Hong WK (2018) Enhanced production of carotenoids using a thraustochytrid microalgal strain containing high levels of docosahexaenoic acid-rich oil. Bioprocess Biosyst Eng 41:355–1370
Prasad P, Savyasachi S, Prasanna Anjaneya Reddy L, Sreedhar RV (2019) Physico-chemical characterization, profiling of total lipids and triacylglycerol molecular species of omega-3 fatty acid rich B. arvensis seed oil from India. J Oleo Sci 68:209–223
Rajvanshi PK, Arya M, Rajasekharan R (2017) The stress-regulatory transcription factors Msn2 and Msn4 regulate fatty acid oxidation in budding yeast. J Biol Chem 292:18628–18643
Rhodes L, Smith J, Tervit R, Roberts R, Adamson J, Adams S, Decker M (2006) Cryopreservation of economically valuable marine micro-algae in the classes Bacillariophyceae, Chlorophyceae, Cyanophyceae, Dinophyceae, Haptophyceae, Prasinophyceae, and Rhodophyceae. Cryobiology 52:152–156
Rosa SM, Soria MA, Vélez CG, Galvagno MA (2010) Improvement of a two-stage fermentation process for docosahexaenoic acid production by Aurantiochytrium limacinum SR21 applying statistical experimental designs and data analysis. Bioresour Technol 101:2367–2374
Scarbrough C, Wirschell M (2016) Comparative analysis of cryopreservation methods in Chlamydomonas reinhardtii. Cryobiology 73:291–295
Ścieszka S, Klewicka E (2019) Algae in food: a general review. Crit Rev Food Sci Nutr 59:3538–3547
Stacey GN, Day JG (2014) Putting cells to sleep for future science. Nat Biotechnol 32:320–322
Taylor R, Fletcher RL (1998) Cryopreservation of eukaryotic algae - a review of methodologies. J Appl Phycol 10:481–501
Transparency market research. Microalgae-based Products Market. https://www.transparencymarketresearch.com/microalgae-based-products-market.html. Accessed 17 August 2020
Trevathan-Tackett SM, Treby S, Gleason FH, Macreadie PI, Loke S (2018) Cryopreservation methods are effective for long-term storage of Labyrinthula cultures. Dis Aquat Org 130:65–70
Unagul P, Suetrong S, Preedanon S, Klaysuban A, Gundool W, Suriyachadkun C, Sakayaroj J (2017) Isolation, fatty acid profiles and cryopreservation of marine thraustochytrids from mangrove habitats in Thailand. Bot Mar 60:363–379
Velmurugan N, Sathishkumar Y, Yim SS, Yang SL, Park MS, Yang JW, Jeong KJ (2014) Study of cellular development and intracellular lipid bodies accumulation in the thraustochytrid Aurantiochytrium sp. KRS101. Bioresour Technol 161:149–154
Yang D, Li W (2016) Methanol-promoted lipid remodelling during cooling sustains cryopreservation survival of Chlamydomonas reinhardtii. PLoS One 11:e0146255
Acknowledgements
We greatly acknowledge the Director, Council of Scientific and Industrial Research (CSIR)-Central Food Technological Research Institute (CFTRI), Mysore, for his kind support and encouragement throughout the study.
Funding
The author AD is a recipient of the award of Senior Research Fellowship from the University Grants Commission, New Delhi. The funding of the work was provided by the Council of Scientific and Industrial Research (CSIR)-Central Food Technological Research Institute (CFTRI).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PDF 252 kb)
Rights and permissions
About this article
Cite this article
Dalmia, A., Tumaney, A.W. Optimisation and evaluation of cryopreservation method for Aurantiochytrium limacinum. J Appl Phycol 33, 869–878 (2021). https://doi.org/10.1007/s10811-020-02346-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02346-3