Abstract
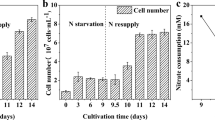
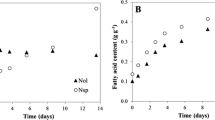
Oleaginous microalgae can accumulate large amounts of storage lipids that have many potential applications such as in the production of biodiesel and health products. All of the energy for the synthesis of lipids in microalgae is derived from photosynthesis. To date, however, the quantitative relationship between photosynthesis and lipid accumulation rate in microalgae is still unclear. In this study, Nannochloropsis sp. was selected to explore this relationship by investigating changes in lipid accumulation, photosynthetic efficiency, and the electron transport chain under nutrients limitation and replenishment. The results of the study demonstrated that lipids were the main form of storage of carbon and energy for this microalga. The alternative electron transport chain played an important role in photo-protection and lipid accumulation. The photosynthetic efficiency of this microalga showed a strong correlation with lipid accumulation rate (R2 = 0.959), with higher photosynthetic efficiency translating to higher lipid accumulation rates during the lipid accumulation stage of the growth cycle. The results of the present study indicated that nutrient depletion might not be the best signal to understand when microalgae begin to accumulate oil, as it appears that photosynthetic efficiency also has a large role in the rate of oil accumulation. Additionally, the study shows that excessive light might not be needed in later stages of growth for lipid accumulation, which in turn would alleviate the damage to microalgae caused by strong light making large-scale cultivation more cost-effective.






Similar content being viewed by others
References
Adarme-Vega TC, Lim DK, Timmins M, Vernen F, Li Y, Schenk PM (2012) Microalgal biofactories: a promising approach towards sustainable omega-3 fatty acid production. Microb Cell Factories 11:96
Benvenuti G, Bosma R, Cuaresma M, Janssen M, Barbosa MJ, Wijffels RH (2015) Selecting microalgae with high lipid productivity and photosynthetic activity under nitrogen starvation. J Appl Phycol 27:1425–1431
Berges JA, Charlebois DO, Mauzerall DC, Falkowski PG (1996) Differential effects of nitrogen limitation on photosynthetic efficiency of photosystems I and II in microalgae. Plant Physiol 110:689–696
Boussiba S, Fan L, Vonshak A (1992) Enhancement and determination of astaxanthin accumulation in green alga Haematococcus pluvialis. Methods Enzymol 213:386–391
Breuer G, Lamers PP, Martens DE, Draaisma RB, Wijffels RH (2012) The impact of nitrogen starvation on the dynamics of triacylglycerol accumulation in nine microalgae strains. Bioresour Technol 124:217–226
Breuer G, Martens DE, Draaisma RB, Wijffels RH, Lamers PP (2015) Photosynthetic efficiency and carbon partitioning in nitrogen-starved Scenedesmus obliquus. Algal Res 9:254–262
Cardol P, Forti G, Finazzi G (2011) Regulation of electron transport in microalgae. BBA-Bioenergetics 1807:912–918
Dong H, Williams E, Wang D, Xie Z, Rc H, Jenck A, Halden R, Li J, Chen F, Place AR (2013) Responses of Nannochloropsis oceanica IMET1 to long-term nitrogen starvation and recovery. Plant Physiol 162:1110–1126
Dubois M, Gilles KA, Hamilton JK, Rebers P, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Gao Y, Yang M, Wang C (2013) Nutrient deprivation enhances lipid content in marine microalgae. Bioresour Technol 147:484–491
He Q, Yang H, Wu L, Hu C (2015) Effect of light intensity on physiological changes, carbon allocation and neutral lipid accumulation in oleaginous microalgae. Bioresour Technol 191:219–228
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008) Microalgal triacylglycerols as feedstocks for biofuel production: perspectives and advances. J Plant 54:621–639
Jiang Y, Yoshida T, Quigg A (2012) Photosynthetic performance, lipid production and biomass composition in response to nitrogen limitation in marine microalgae. Plant Physiol Biochem 54:70–77
Khozin-Goldberg I, Shrestha P, Cohen Z (2005) Mobilization of arachidonyl moieties from triacylglycerols into chloroplastic lipids following recovery from nitrogen starvation of the microalga Parietochloris incisa. BBA Mol Cell Biol 1738:63–71
Kim YJ, Lee HM, Wang Y, Wu J, Kim SG, Kang KY, Park KH, Kim YC, Choi IS, Agrawal GK (2013) Depletion of abundant plant Rubisco protein using the protamine sulfate precipitation method. Proteomics 13:2176–2179
Klok AJ, Martens DE, Wijffels RH, Lamers PP (2013) Simultaneous growth and neutral lipid accumulation in microalgae. Bioresour Technol 134:233–243
Klok AJ, Lamers PP, Martens DE, Draaisma RB, Wijffels RH (2014) Edible oils from microalgae: insights in TAG accumulation. Trends Biotechnol 32:521–528
Li Y, Han D, Sommerfeld M, Hu Q (2011) Photosynthetic carbon partitioning and lipid production in the oleaginous microalga Pseudochlorococcum sp. (Chlorophyceae) under nitrogen-limited conditions. Bioresour Technol 102:123–129
Li T, Wan L, Li A, Zhang C (2013) Responses in growth, lipid accumulation, and fatty acid composition of four oleaginous microalgae to different nitrogen sources and concentrations. Chin J Oceanol Limnol 31:1306–1314
Li T, Xu J, Gao B, Xiang W, Li A, Zhang C (2016) Morphology, growth, biochemical composition and photosynthetic performance of Chlorella vulgaris (Trebouxiophyceae) under low and high nitrogen supplies. Algal Res 16:481–491
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Masojídek J, Torzillo G, Kopecký J, Koblížek M, Nidiaci L, Komenda J, Lukavská A, Sacchi A (2000) Changes in chlorophyll fluorescence quenching and pigment composition in the green alga Chlorococcum sp. grown under nitrogen deficiency and salinity stress. J Appl Phycol 12:417–426
Masojídek J, Koblížek M, Torzillo G (2004) Photosynthesis in microalgae. In: Richmond A (ed) Handbook of microalgal culture: biotechnology and applied phycology. Blackwell, Oxford, pp 20–39
Melis A (2009) Solar energy conversion efficiencies in photosynthesis: minimizing the chlorophyll antennae to maximize efficiency. Plant Sci 177:272–280
Najafabadi HA, Malekzadeh M, Jalilian F, Vossoughi M, Pazuki G (2015) Effect of various carbon sources on biomass and lipid production of Chlorella vulgaris during nutrient sufficient and nitrogen starvation conditions. Bioresour Technol 180:311–317
Pancha I, Chokshi K, George B, Ghosh T, Paliwal C, Maurya R, Mishra S (2014) Nitrogen stress triggered biochemical and morphological changes in the microalgae Scenedesmus sp. CCNM 1077. Bioresour Technol 156:146–154
Rodolfi L, Chini Zittelli G, Bassi N, Padovani G, Biondi N, Bonini G, Tredici MR (2009) Microalgae for oil: strain selection, induction of lipid synthesis and outdoor mass cultivation in a low-cost photobioreactor. Biotechnol Bioeng 102:100–112
Siaut M, Cuiné S, Cagnon C, Fessler B, Nguyen M, Carrier P, Beyly A, Beisson F, Triantaphylidès C, Li-Beisson Y (2011) Oil accumulation in the model green alga Chlamydomonas reinhardtii: characterization, variability between common laboratory strains and relationship with starch reserves. BMC Biotechnol 11:7
Sibi G (2015) Inhibition of lipase and inflammatory mediators by Chlorella lipid extracts for antiacne treatment. J Adv Pharm Technol Res 6:7–12
Simionato D, Block MA, La Rocca N, Jouhet J, Maréchal E, Finazzi G, Morosinotto T (2013) The response of Nannochloropsis gaditana to nitrogen starvation includes de novo biosynthesis of triacylglycerols, a decrease of chloroplast galactolipids, and reorganization of the photosynthetic apparatus. Eukaryot Cell 12:665–676
Solovchenko A (2012) Physiological role of neutral lipid accumulation in eukaryotic microalgae under stresses. Russ J Plant Physiol 59:167–176
Turpin DH (1991) Effects of inorganic N availability on algal photosynthesis and carbon metabolism. J Phycol 27:14–20
Vello V, Chu W-L, Lim P-E, Majid NA, Phang S-M (2018) Metabolomic profiles of tropical Chlorella species in response to physiological changes during nitrogen deprivation. J Appl Phycol 30:3131–3151
Wagner H, Jakob T, Wilhelm C (2006) Balancing the energy flow from captured light to biomass under fluctuating light conditions. New Phytol 169:95–108
White S, Anandraj A, Bux F (2011) PAM fluorometry as a tool to assess microalgal nutrient stress and monitor cellular neutral lipids. Bioresour Technol 102:1675–1682
Wilhelm C, Selmar D (2011) Energy dissipation is an essential mechanism to sustain the viability of plants: the physiological limits of improved photosynthesis. J Plant Physiol 168:79–87
Yang X, Li Y, Li Y, Ye D, Yuan L, Sun Y, Han D, Hu Q (2019) Solid matrix-supported supercritical CO2 enhances extraction of γ-linolenic acid from the cyanobacterium Arthrospira (Spirulina) platensis and bioactivity evaluation of the molecule in Zebrafish. Mar Drugs 17:203
Zhang Y, Wu H, Yuan C, Li T, Li A (2019) Growth, biochemical composition, and photosynthetic performance of Scenedesmus acuminatus during nitrogen starvation and resupply. J Appl Phycol 31:2797–2809
Acknowledgments
Thanks to W. Tyler Mehler for assistance with the language of the manuscript.
Funding
This work was supported with funds from The National Natural Science Foundation of China (31602182) and The PhD Start-up Fund of Natural Science Foundation of Guangdong Province (2016A030310016).
Author information
Authors and Affiliations
Contributions
Tao Li designed the study, performed the experiments, analyzed the results, and drafted the manuscript. Chaojie Yuan and Weinan Wang carried out cultivation and the biochemical composition analysis of Nannochloropsis sp. Ying Zhang performed the statistical analysis and helped to draft the manuscript. Jin Xu participated in the isolation of Nannochloropsis sp. Helong Zheng and Wenzhou Xiang assisted in the determination of photosynthetic efficiency. Aifen Li took part in designing the study, coordinating the study, and assisted with revisions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Figure S1
Nitrate and phosphate concentration in culture medium A. nitrate concentration; B. phosphate concentration. Ten mL of the culture was obtained and centrifuged at 8000 rpm for 10 min. The supernatant was collected and then filtered by using a piece of 0.22 μM filter membrane. The nitrate concentration was determined in this filtered sample using an AutoAnalyzer3 (Bran-Luebbe, Norderstedt, Germany). Analysis of phosphate concentration in the medium was done in accordance with the instruction of its respected kits. In short, PhosVer 3 phosphate reagent (21060–69) was added to each sample to determine phosphate concentrations, respectively. The collected filtrate for phosphate analysis was shaken for 15 s and allowed to rest for 2 min, while the collected filtrate for phosphate was shaken for 30 s with a 15 min rest period. Phosphate concentrations were determined using a Hach DR2700 (Hach, Loveland, USA) (JPG 1329 kb)
Rights and permissions
About this article
Cite this article
Li, T., Wang, W., Yuan, C. et al. Linking lipid accumulation and photosynthetic efficiency in Nannochloropsis sp. under nutrient limitation and replenishment. J Appl Phycol 32, 1619–1630 (2020). https://doi.org/10.1007/s10811-020-02056-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-020-02056-w