Abstract
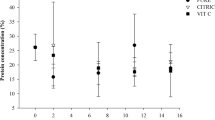
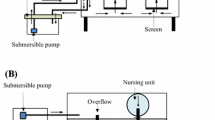
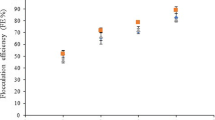
The effects of the cell separation method and the biomass processing of Nannochloropsis oculata were evaluated with respect to the cultivation performance of rotifers (Brachionus sp.). The algal culture was harvested by centrifugation or flocculation, and the biomass separation efficiency and the concentration factor were determined. The biomass was processed by chilling, freezing, heat-drying, or freeze-drying; and the cell integrity, resuspension percentage, and sedimentation profile of the diets were evaluated. The zootechnical performance (maximum rotifer density, growth rate, maximum egg production, fecundity, and feed ingestion rate) of rotifer cultures that fed the concentrated diets was compared with feeding the fresh culture of N. oculata (control treatment). Centrifugation resulted in 97% separation efficiency and a concentration factor of 578 and flocculation followed by centrifugation showed an efficiency of ~ 100% and concentration factor of 444. The harvesting technique did not have a significant influence on the characteristics of the diets and also on the zootechnical performance of the rotifers. The characteristics of the chilled concentrates (cell integrity, resuspension percentage, and sedimentation profiles) were similar to the control treatment, and the rotifers fed on those diets had the highest growth performances. Rotifers fed on frozen diets presented the worst performances. The heat-dried diets presented the lowest cell viability percentages, lowest resuspension percentages, and higher sedimentation rates. The feed ingestion rates of these diets were the lowest, resulting on inferior growth performance of the rotifers. In summary, both harvesting methods could be used, and chilling is the most recommended method for processing N. oculata biomass.


Similar content being viewed by others
References
Albentosa M, Pérez-Camacho A, Labarta U, Fernández-Reiriz MJ (1997) Evaluation of freeze-dried microalgal diets for the seed culture of Ruditapes decussatus using physiological and biochemical parameters. Aquaculture 154:305–321
Alver MO, Alfredsen JA, Øie G, Storøy W, Olsen Y (2010) Automatic control of growth and density in rotifer cultures. Aquac Eng 43:6–13
Arney B, Liu W, Forster IP, McKinley RS, Pearce CM (2015) Feasibility of dietary substitution of live microalgae with spray-dried Schizochytrium sp. or Spirulina in the hatchery culture of juveniles of the Pacific geoduck clam (Panopea generosa). Aquaculture 444:117–133
Balompapueng M, Hagiwara A, Nishi A, Imaizumi K, Hirayama K (1997) Resting egg formation of the rotifer Brachionus plicatilis using a semi-continuous culture method. Fish Sci 63:236–241
Benemann J (2013) Microalgae for biofuels and animal feeds. Energies 6:5869–5886
Borowitzka MA (1997) Microalgae for aquaculture: opportunities and constraints. J Appl Phycol 9:393–401
Camacho-Rodríguez J, Cerón-García MC, Macías-Sánchez MD, Fernández-Sevilla JM, López-Rosales L, Molina-Grima E (2016) Long-term preservation of concentrated Nannochloropsis gaditana cultures for use in aquaculture. J Appl Phycol 28:299–312
Cañavate JP, Fernández-Díaz C (2001) Pilot evaluation of freeze-dried microalgae in the mass rearing of gilthead seabream (Sparus aurata) larvae. Aquaculture 193:257–269
Conceição LEC, Yúfera M, Makridis P, Morais S, Dinis MT (2010) Live feeds for early stages of fish rearing. Aquac Res 41:613–640
Coutteau P, Sorgeloos P (1992) Use of algal substitutes and the requirement for live algae in the hachery of bivalve molluscs: an international survey. J Shellfish Res 11:467–476
D’Souza FML, Lecossois D, Heasman MP, Diemar JA, Jackson CJ, Pendrey RC (2000) Evaluation of centrifuged microalgae concentrates as diets for Penaeus monodon Fabricius larvae. Aquac Res 31:661–670
Faulk CK, Holt GJ (2005) Advances in rearing cobia Rachycentron canadum larvae in recirculating aquaculture systems: live prey enrichment and greenwater culture. Aquaculture 249:231–243
Galvão MSN, Yamanaka N, Tanji S (1996) Preservação de duas microalgas Nannochloropsis oculata e Tetraselmis tetrathele por resfriamento e congelamento. Bol Inst Pesca 23:1–11
Guevara M, Bastardo L, Cortez R, Arredondo-Vega B, Romero L, Gomez P (2011) Pastas de Rhodomonas salina (Cryptophyta) como alimento para Brachionus plicatilis (Rotifera). Rev Biol Trop 59:1503–1515
Hair C, Kaure T, Southgate P, Pickering T (2011) Potential breakthrough in hatchery culture of sandfish Holothuria scabra by using algal concentrate as food. SPC Beche-de-mer Inf Bull 31:60–61
Halim R, Danquah MK, Webley PA (2012) Extraction of oil from microalgae for biodiesel production: a review. Biotechnol Adv 30:709–732
Hamada K, Hagiwara A, Hirata K (1993) Use of preserved diet for rotifer Brachionus plicatilis resting egg formation. Nippon Suisan Gakkaishi 59:85–91
Haver VL, Nayar S (2017) Polyelectrolyte flocculants in harvesting microalgal biomass for food and feed applications. Algal Res 24:167–180
Heasman M, Diemar J, Connor WO et al (2000) Development of extended shelf-life microalgae concentrate diets harvested by centrifugation for bivalve molluscs - a summary. Aquac Res 31:637–659
Low C, Toledo MI (2015) Assessment of the shelf life of Nannochloropsis oculata flocculates stored at different temperatures. Lat Am J Aquat Res 43:315–321
Milione M, Zeng C (2007) The effects of algal diets on population growth and egg hatching success of the tropical calanoid copepod, Acartia sinjiensis. Aquaculture 273:656–664
Molina-Grima E, Acién-Fernández FG, Robles-Medina A (2013) Downstream processing of cell mass and products. In: Richmond A, Hu Q (eds) Handbook of microalgal culture: applied phycology and biotechnology, 2nd edn. Wiley, London, pp 267–309
Muller-Feuga A (2000) The role of microalgae in aquaculture: situation and trends. J Appl Phycol 12:527–534
Navarro N, Yúfera M (1998) Influence of the food ration and individual density on production efficiency of semicontinuous cultures of Brachionus-fed microalgae dry powder. Hydrobiologia 387:483–487
Navarro N, Yúfera M, García-Gallego M (2001) Use of freeze-dried microalgae for rearing gilthead seabream, Sparus aurata L., larvae. II. Biochemical composition. Hydrobiologia 452:69–77
Neori A (2010) “Green water” microalgae: the leading sector in world aquaculture. J Appl Phycol 23:143–149
Oliveira EG, Duarte JH, Moraes K, Crexi VT, Pinto LAA (2010) Optimisation of Spirulina platensis convective drying: evaluation of phycocyanin loss and lipid oxidation. Int J Food Sci Technol 45:1572–1578
Ponis E, Robert R, Parisi G (2003) Nutritional value of fresh and concentrated algal diets for larval and juvenile Pacific oysters (Crassostrea gigas). Aquaculture 221:491–505
Rehberg-Haas S, Meyer S, Lippemeier S, Schulz C (2015) A comparison among different Pavlova sp. products for cultivation of Brachionus plicatilis. Aquaculture 435:424–430
Rojo-Cebreros A, Morales-Plascencia M, Ibarra-Castro L, Martinez-Brown JM, Medina-Jasso MA (2016) Floculacion de Nannochloropsis sp. inducida por hidroxido de sodio: eficiencia de floculacion, efecto sobre la viabilidad microalgal y su uso como alimento para rotiferos. Lat Am J Aquat Res 44:662–670
Ryckebosch E, Muylaert K, Eeckhout M, Ruyssen T, Foubert I (2011) Influence of drying and storage on lipid and carotenoid stability of the microalga Phaeodactylum tricornutum. J Agric Food Chem 59:11063–11069
Sales R, Abreu PC (2015) Use of natural pH variation to increase the flocculation of the marine microalgae Nannochloropsis oculata. Appl Biochem Biotechnol 175:2012–2019
Sales R, Mélo RCS, de Moraes RM, da Silva RCS, Cavalli RO, do Amaral Ferraz Navarro DM, de Souza Santos LP (2016) Production and use of a flocculated paste of Nannochloropsis oculata for rearing newborn seahorse Hippocampus reidi. Algal Res 17:142–149. doi:
Seychelles LH, Audet C, Tremblay R, Fournier R, Pernet F (2009) Essential fatty acid enrichment of cultured rotifers (Brachionus plicatilis, Müller) using frozen-concentrated microalgae. Aquac Nutr 15:431–439
Southgate PC, Beer AC, Ngaluafe P (2016) Hatchery culture of the winged pearl oyster, Pteria penguin, without living micro-algae. Aquaculture 451:121–124
Uduman N, Qi Y, Danquah MK, Forde GM, Hoadley A (2010) Dewatering of microalgal cultures: a major bottleneck to algae-based fuels. J Renew Sustain Ener 2:1–15
Walne PR (1956) Experiments in the large-scale culture of the larvae of Ostrea edulis. Fish Invest 25:1–53
Welladsen H, Kent M, Mangott A, Li Y (2014) Shelf-life assessment of microalgae concentrates: effect of cold preservation on microalgal nutrition profiles. Aquaculture 430:241–247
Yamasaki S, Tanabe K, Hirata H (1989) Efficiency of chilled and frozen Nannochloropsis sp.(marine chlorella) for culture of rotifer. Mem Fac Fish Kagoshima Univ 38:77–82
Yoshimura K, Tanaka K, Yoshimatsu T (2003) A novel culture system for the ultra-high-density production of the rotifer, Brachionus rotundiformis - a preliminary report. Aquaculture 227:165–172
Yoshimura K, Usuki K, Yoshimatsu T, Kitajima C, Hagiwara A (1997) Recent development of a high density mass culture system for the rotifer Brachionus rotundiformis Tschugunoff. Hydrobiologia 358:139–144
Yúfera M, Navarro N (1995) Population growth dynamics of the rotifer Brachionus plicatilis cultured in non-limiting food condition. Hydrobiologia 313–314:399–405
Yúfera M, Pascual E (1985) Effects of algal food concentration on feeding and ingestion rates of Brachionus plicatilis in mass culture. Hydrobiologia 122:181–187
Funding
This study was supported by the MCTIC/UFSC/FAPEU (Proc. 01200.004541/2015-16) and by CAPES (no. 16 Ciências do Mar 2 N° 43/2013). The first author received a PhD scholarship from the Brazilian Federal Agency for Support and Evaluation of Graduate Education (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior; CAPES), Finance Code 001, and the last author grant from the National Council for Scientific and Technological Development (CNPq 306078/2017-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sales, R., Derner, R.B. & Tsuzuki, M.Y. Effects of different harvesting and processing methods on Nannochloropsis oculata concentrates and their application on rotifer Brachionus sp. cultures. J Appl Phycol 31, 3607–3615 (2019). https://doi.org/10.1007/s10811-019-01877-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-01877-8