Abstract
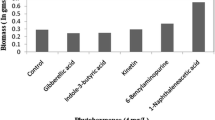
Demand for eicosapentaenoic acid (EPA) is growing rapidly due to proven therapeutic potential. Microalgae such as Monodopsis subterranea are considered important and sustainable source of eicosapentaenoic acid and require further enhancement in productivity. The present study investigated the role of auxins and cytokinin for enhanced EPA productivity in M. subterranea. The study evaluated the effect of these phytohormones on biomass, fatty acid composition, EPA productivity, and total fatty acid (TFA) productivity through dose-dependent responses at various growth phases. Treatment hierarchy in terms of stimulatory effect on EPA productivity is indole-3-acetic acid (IAA) > indole-3-butyric acid (IBA) > 6-benzyl amino purine (6BAP) > naphthalene acetic acid (NAA). Supplementation of 100 μM (IAA) leads to a maximum increase in EPA productivity (2.5-fold) at late-exponential phase. Results show that 100 μM IBA yields maximum enhancement in EPA (58%) and TFA content (88%) and resulted in increased productivity by 1.8-fold and 2.1-fold, respectively, at mid-exponential phase. IBA at 100 μM also leads to the highest biomass productivity of 2.2-fold at late stationary phase. Although the addition of the cytokinin 6-BAP at 1 μM showed a marginal increase in biomass accumulation, biomass exhibited an inverse relation with increase in 6BAP concentration. Statistical analysis using principal components analysis demonstrated stimulatory action of IAA and IBA on biomass and EPA productivity at late-exponential phase.




Similar content being viewed by others
References
Adarme-Vega TC, Lim DK, Timmins M, Vernen F, Li Y, Schenk PM (2012) Microalgal biofactories: a promising approach towards sustainable omega-3 fatty acid production. Microb Cell Factories 11:96
Adarme-Vega TC, Thomas-Hall SR, Schenk PM (2014) Towards sustainable sources for omega-3 fatty acids production. Curr Opin Biotechnol 26:14–18
Bartel B, LeClere S, Magidin M, Zolman BK (2001) Inputs to the active indole-3-acetic acid pool: de novo synthesis, conjugate hydrolysis, and indole-3-butyric acid β-oxidation. J Plant Growth Regul 20:198–216
Blanc G, Duncan G, Agarkova I, Borodovsky M, Gurnon J, Kuo A, Lindquist E, Lucas S, Pangilinan J, Polle J, Salamov A (2010) The Chlorella variabilis NC64A genome reveals adaptation to photosymbiosis, coevolution with viruses, and cryptic sex. Plant Cell 22:2943–2955
Camacho-Rodríguez J, Cerón-García MC, González-López CV, Fernández-Sevilla JM, Contreras-Gómez A, Molina-Grima E (2013) A low-cost culture medium for the production of Nannochloropsis gaditana biomass optimized for aquaculture. Bioresour Technol 144:57–66
Carimi F, Terzi M, De Michele R, Zottini M, Schiavo FL (2013) High levels of the cytokinin BAP induce PCD by accelerating senescence. Plant Sci J 166:963–969
Castoldi AF, Johansson C, Onishchenko N, Coccini T, Roda E, Vahter M, Ceccatelli S, Manzo L (2005) Human developmental neurotoxicity of methylmercury: impact of variables and risk modifiers. Regul Toxicol Pharmacol 51:201–214
Cepák V, Přibyl P, Kohoutková J, Kaštánek P (2014) Optimization of cultivation conditions for fatty acid composition and EPA production in the eustigmatophycean microalga Trachydiscus minutus. J Appl Phycol 26:181–190
Cerón-García MC, Fernández-Sevilla JM, Sánchez-Mirón A, García-Camacho F, Contreras-Gómez A, Molina-Grima E (2013) Mixotrophic growth of Phaeodactylum tricornutum on fructose and glycerol in fed-batch and semi-continuous modes. Bioresour Technol 147:569–576
Choudhary AK, Sunojkumar P, Mishra G (2017) Fatty acid profiling and multivariate analysis in the genus Leucas reveals its nutritional, pharmaceutical and chemotaxonomic significance. Phytochemistry 143:72–80
Cohen Z (1994) Production potential of eicosapentaenoic acid by Monodus subterraneus. J Am Oil Chem Soc 71:941–945
Colombo J, Koletzko B, Lampl M (eds) (2017) Recent research in nutrition and growth, 89th Nestlé Nutrition Institute Workshop, Dubai. Karger, Basel
Czerpak R, Bajguz A (1997) Stimulatory effect of auxins and cytokinins on carotenes, with differential effects on xanthophylls in the green alga Chlorella pyrenoidosa Chick. Acta Soc Bot Pol 66:41–46
Dao GH, Wu GX, Wang XX, Zhuang LL, Zhang TY, Hu HY (2018) Enhanced growth and fatty acid accumulation of microalgae Scenedesmus sp. LX1 by two types of auxin. Bioresour Technol 247:561–567
Delbarre A, Muller P, Imhoff V, Guern J (1996) Comparison of mechanisms controlling uptake and accumulation of 2,4-dichlorophenoxy acetic acid, naphthalene-1-acetic acid, and indole-3-acetic acid in suspension-cultured tobacco cells. Planta 198:532–541
Farag MA, Al-Mahdy DA, Meyer A, Westphal H, Wessjohann LA (2017) Metabolomics reveals biotic and abiotic elicitor effects on the soft coral Sarcophyton ehrenbergi terpenoid content. Sci Rep 7:648
Flasiński M, Hąc-Wydro K (2014) Natural vs synthetic auxin: studies on the interactions between plant hormones and biological membrane lipids. Environ Res 133:123–134
Galun E (2010) Phytohormones and patterning: the role of hormones in plant architecture. World Scientific Publishing Company, Singapore
Garcia-Mier L, Jimenez-Garcia SN, Guevara-González RG, Feregrino-Perez AA, Contreras-Medina LM, Torres-Pacheco I (2015) Elicitor mixtures significantly increase bioactive compounds, antioxidant activity, and quality parameters in sweet bell pepper. J Chem 2015:269296
Gill I, Valivety R (1997) Polyunsaturated fatty acids, part 1: occurrence, biological activities and applications. Trends Biotechnol 15:401–409
González-López CV, Cerón-García MC, Fernández-Sevilla JM, González-Céspedes AM, Camacho-Rodríguez J, Molina-Grima E (2013) Medium recycling for Nannochloropsis gaditana cultures for aquaculture. Bioresour Technol 129:430–438
Han X, Zeng H, Bartocci P, Fantozzi F, Yan Y (2018) Phytohormones and effects on growth and metabolites of microalgae: a review. Fermentation 4:25
Higgs GA (1986) The role of eicosanoids in inflammation. Prog Lipid Res 25:555–561
Hunt RW, Chinnasamy S, Das KC (2011) The effect of naphthalene-acetic acid on biomass productivity and chlorophyll content of green algae, coccolithophore, diatom, and cyanobacterium cultures. Appl Biochem Biotechnol 164:1350–1365
Jiao K, Chang J, Zeng X, Ng IS, Xiao Z, Sun Y, Tang X, Lin L (2017) 5-Aminolevulinic acid promotes arachidonic acid biosynthesis in the red microalga Porphyridium purpureum. Biotechnol Biofuels 10:168
Jusoh M, Loh SH, Chuah TS, Aziz A, San-Cha T (2015) Indole-3-acetic acid (IAA) induced changes in oil content, fatty acid profiles and expression of four fatty acid biosynthetic genes in Chlorella vulgaris at early stationary growth phase. Phytochemistry 111:65–71
Khozin-Goldberg I, Cohen Z (2006) The effect of phosphate starvation on the lipid and fatty acid composition of the fresh water eustigmatophyte Monodus subterraneus. Phytochemistry 67:696–701
Kokkiligadda S, Pandey B, Ronda SR (2017) Effect of plant growth regulators on production of alpha-linolenic acid from microalgae Chlorella pyrenoidosa. Sādhanā 42:1821–1824
Liang Y, Beardall J, Heraud P (2006) Effect of UV radiation on growth, chlorophyll fluorescence and fatty acid composition of Phaeodactylum tricornutum and Chaetoceros muelleri (Bacillariophyceae). Phycologia 45:605–615
Liu J, Song Y, Liu Y, Ruan R (2015) Optimization of growth conditions toward two-stage cultivation for lipid production of Chlorella vulgaris. Environ Prog Sustain Energy 34:1801–1807
Liu T, Liu F, Wang C, Wang Z, Li Y (2017) The boosted biomass and lipid accumulation in Chlorella vulgaris by supplementation of synthetic phytohormone analogs. Bioresour Technol 232:44–52
Lu Y, Xu J (2015) Phytohormones in microalgae: a new opportunity for microalgal biotechnology? Trends Plant Sci 20:273–282
Lu C, Fernández FA, Guerrero EC, Hall DO, Grima EM (2002) Overall assessment of Monodus subterraneus cultivation and EPA production in outdoor helical and bubble column reactors. J Appl Phycol 14:331–342
Lukeš M, Giordano M, Prášil O (2017) The effect of environmental factors on fatty acid composition of Chromera velia (Chromeridae). J Appl Phycol 29:1791–1799
Maitra S, Yan J (2008) Principle component analysis and partial least squares: two dimension reduction techniques for regression. Applying Multivariate Statistical Models. Casualty Actuarial Society Discussion Paper Program 79–90
Meireles LA, Guedes AC, Malcata FX (2003) Increase of the yields of eicosapentaenoic and docosahexaenoic acids by the microalga Pavlova lutheri following random mutagenesis. Biotechnol Bioeng 81:50–55
Moffitt CM, Cajas-Cano L (2014) Blue growth: the 2014 FAO state of world fisheries and aquaculture. Fisheries 39:552–553
Mozaffarian D, Wu JH (2011) Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol 58:2047–2067
Nichols BW, Appleby RS (1969) The distribution and biosynthesis of arachidonic acid in algae. Phytochemistry 8:1907–1915
Pal D, Khozin-Goldberg I, Cohen Z, Boussiba S (2011) The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl Microbiol Biotechnol 90:1429–1441
Parsaeimehr A, Mancera-Andrade EI, Robledo-Padilla F, Iqbal HM, Parra-Saldivar R (2017) A chemical approach to manipulate the algal growth, lipid content and high-value alpha-linolenic acid for biodiesel production. Algal Res 26:312–322
Piotrowska-Niczyporuk A, Bajguz A (2014) The effect of natural and synthetic auxins on the growth, metabolite content and antioxidant response of green alga Chlorella vulgaris (Trebouxiophyceae). Plant Growth Regul 73:57–66
Qiang HU, Zheungu H, Cohen Z, Richond A (1997) Enhancement of eicosapentaenoic acid (EPA) and γ-linolenic acid (GLA) production by manipulating algal density of outdoor cultures of Monodus subterraneus (Eustigmatophyta) and Spirulina platensis (Cyanobacteria). Eur J Phycol 32:81–86
Qiao H, Cong C, Sun C, Li B, Wang J, Zhang L (2016) Effect of culture conditions on growth, fatty acid composition and DHA/EPA ratio of Phaeodactylum tricornutum. Aquaculture 452:311–317
Renuka N, Guldhe A, Singh P, Bux F (2018) Combined effect of exogenous phytohormones on biomass and lipid production in Acutodesmus obliquus under nitrogen limitation. Energy Convers Manag 168:522–528
Romanenko KO, Kosakovskaya IV, Romanenko PO (2016) Phytohormones of microalgae: biological role and involvement in the regulation of physiological processes. Pt II. Cytokinins and Gibberellins. Int J Algae 18:179–201
Salama ES, Kabra AN, Ji MK, Kim JR, Min B, Jeon BH (2014) Enhancement of microalgae growth and fatty acid content under the influence of phytohormones. Bioresour Technol 172:97–103
Samuelsen TA, Oterhals Å, Kousoulaki K (2018) High lipid microalgae (Schizochytrium sp.) inclusion as a sustainable source of n-3 long-chain PUFA in fish feed—effects on the extrusion process and physical pellet quality. Anim Feed Sci Technol 236:14–28
Shah MR, Lutzu GA, Alam A, Sarker P, Kabir Chowdhury MA, Parsaeimehr A, Liang Y, Daroch M (2018) Microalgae in aquafeeds for a sustainable aquaculture industry. J Appl Phycol 30:197–213
Simopoulos AP, Bazán NG (eds) (2009) Omega-3 fatty acids, the brain and retina. Karger, Basel
Stevanato P, Broccanello C, Moliterni V, Mandolino G, Barone V, Lucini L, Baglieri A (2018) Innovative approaches to evaluate sugar beet responses to changes in sulfate availability. Front Plant Sci:9–14
Stirk WA, Ördög V, Novák O, Rolčík J, Strnad M, Bálint P, Van Staden J (2013) Auxin and cytokinin relationships in 24 microalgal strains. J Phycol 49:459–467
Tatsuzawa H, Takizawa E (1995) Changes in lipid and fatty acid composition of Pavlova lutheri. Phytochemistry 40:397–400
Tuteja N, Sopory SK (2008) Chemical signaling under abiotic stress environment in plants. Plant Signal Behav 3:525–536
Van der Voort MPJ, Spruijt J, Potters JI, Elissen HJH (2017) Socio-economic assessment of algae-based PUFA production, Public Output report of the PUFAChain project, Göttingen, 79 pp, http://www.pufachain.eu
Van Wagenen J, Miller TW, Hobbs S, Hook P, Crowe B, Huesemann M (2012) Effects of light and temperature on fatty acid production in Nannochloropsis salina. Energies 5:731–740
Yu XJ, Sun J, Zheng JY, Sun YQ, Wang Z (2016) Metabolomics analysis reveals 6-benzylaminopurine as a stimulator for improving lipid and DHA accumulation of Aurantiochytrium sp. J Chem Technol Biotechnol 91:1199–1207
Žižková E, Kubeš M, Dobrev PI, Přibyl P, Šimura J, Zahajská L, Motyka V (2016) Control of cytokinin and auxin homeostasis in cyanobacteria and algae. Ann Bot 119:151–166
Bischoff HW, Bold HC (1963) Some algae from Enchanted Rock and related algal species. Phycological studies. University of Texas 4:1-95
Acknowledgments
SA is thankful for CSIR-SRF by Council of Scientific & Industrial Research, Human Resource Development Group (09/045(1562)/2018-EMR-1).
Funding
The authors would like to thank the financial support from the Department of Biotechnology, Ministry of Science and Technology (BT/PR6027/AGII/106/859/2012).
Author information
Authors and Affiliations
Contributions
Both SA and GM carried out the conceptual design of the study and approve the final version of the submitted manuscript; data acquisition, analyses, and manuscript writing were completed by SA with GM contributing for experimental design, data interpretation, and editing the manuscript critically.
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(PDF 397 kb)
Rights and permissions
About this article
Cite this article
Arora, S., Mishra, G. Biochemical modulation of Monodopsis subterranea (Eustigmatophyceae) by auxin and cytokinin enhances eicosapentaenoic acid productivity. J Appl Phycol 31, 3441–3452 (2019). https://doi.org/10.1007/s10811-019-01844-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-01844-3