Abstract
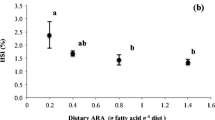
Changes in lipid reserves as a result of supplementing 3.4% Ulva clathrata meal in the diet (based on dry weight of all ingredienta) for Litopenaeus vannamei broodstock were evaluated in a commercial larval laboratory. The profile of fatty acids, sterols, carotenoids, and phenolic compounds in the gonad, hepatopancreas, and muscle of females with unilateral ablation was analyzed. Shrimp fed the Ulva supplemented diet had significantly higher proportions of arachidonic acid (20:4n-6) in the acylglycerides of mature gonads (84% increase) compared with mature controls. In the phospholipids of mature gonads, there was a 30% increase of 18:3n-3, 13% increase of 20:4n-6, and 10% of docosahexaenoic acid (22:6n-3) in Ulva-fed shrimp compared with controls. 22:6n-3 also increased 51% in the lipid reserves of hepatopancreas of Ulva-fed shrimp compared with controls. Carotenoids increased by 43% in the gonads and fivefold in the hepatopancreas of mature females fed Ulva. The proportion of fucosterol and isofucosterol went from undetectable in controls to 0.3 and 0.2%, respectively, in shrimp fed the algae. No significant differences were found in muscle. Phenolic compounds in the gonad were higher in mature gonads compared with immature, but no significant differences were found in relation to Ulva consumption. Carotenoids and phenolic compounds present in Ulva might be acting as antioxidants of highly unsaturated fatty acids (HUFAs), increasing the proportion of 20:4n-6 and 22:6n-3, but other mechanisms associated to these compounds and to fucosterol can be synchronizing oocyte maturation and increasing transport of HUFA during vitellogenesis, and together, they are further impacting the reproductive performance of shrimp.


Similar content being viewed by others
References
Abdul QA, Choi RJ, Jung HA, Choi JS (2016) Health benefit of fucosterol from marine algae: a review. J Sci Food Agric 96:1856–1866
Aguilera-Morales M, Casas-Valdez M, Carrillo-Dominguez B, Gonzalez-Acosta B, Perez-Gil F (2005) Chemical composition and microbiological assays of marine algae Enteromorpha spp. as a potential food source. J Food Compos Anal 18:79–88
Alfaro-Montoya J (2015) The effect of ibuprofen on female and male reproduction of the open thelyca marine shrimp, Litopenaeus. Aquac Res 46:105–116
Boucard CG, Patrois J, Ceccaldi HJ (2004) Exhaustion of lipid reserves in the hepatopancreas of Fenneropenaeus indicus broodstock in relation to successive spawnings. Aquaculture 236:523–537
Cabello-Pasini A, Macías-Carranza V, Abdala R, Korbee N, Figueroa FL (2011) Effect of nitrate concentration and UVR on photosynthesis, respiration, nitrate reductase activity, and phenolic compounds in Ulva rigida (Chlorophyta). J Appl Phycol 23:363–369
Cahu C, Cuzon G, Quazuguel P (1995) Effect of highly unsaturated fatty acids, alpha-tocopherol and ascorbic acid in broodstock diet on egg composition and development of Penaeus indicus. Comp Biochem Physiol 112:417–424
Cardona E, Lorgeoux B, Chim L, Goguenheim J, Le Delliou H, Cahu C (2016) Biofloc contribution to antioxidant defence status, lipid nutrition and reproductive performance of broodstock of the shrimp Litopenaeus stylirostris: consequences for the quality of eggs and larvae. Aquaculture 452:252–262
Carrillo S, López E, Casas M, Ávila E, Castillo R, Carranco M, Calvo C, Perez GF (2008) Potential use of seaweeds in the laying hen ration to improve the quality of n-3 fatty acid enriched eggs. J Appl Phycol 20:721–728
Charniaux-Cotton H (1985) Vitellogenesis and its control in malacostracan Crustacea. Am Zool 25:197–206
Chung MY, Liu CH, Chen YN, Cheng W (2011) Enhancing the reproductive performance of tiger shrimp, Penaeus monodon, by incorporating sodium alginate in the broodstock and larval diets. Aquaculture 312:180–184
Coman GJ, Arnold SJ, Barclay M, Smith DM (2011) Effect of arachidonic acid supplementation on reproductive performance of tank-domesticated Penaeus monodon. Aquac Nutr 17:141–151
Corral-Rosales DC, Palacios E, Ricque-Marie D, Cruz-Suárez LE (2018a) Enhancement of reproductive performance in shrimp Litopenaeus vannamei (Boone, 1931) by supplementation of Ulva clathrata meal in maturation diet in two commercial hatcheries. Aquac Res 49:1053–1059
Corral-Rosales DC, Cruz-Suárez LE, Ricque-Marie D, Rodríguez-Jaramillo C, Palacios E (2018b) Modulation of reproductive exhaustion using Ulva clathrata in Pacific white shrimp Litopenaeus vannamei (Boone, 1931) broodstock during commercial maturation. Aquac Res 49:3711–3722
Dall W, Smith DM, Moore LE (1995) Carotenoids in the tiger prawn Penaeus esculentus during ovarian maturation. Mar Biol 123:435–441
El-Agamey A, Lowe GM, McGarvey DJ, Mortensen A, Phillip DM, Truscott TG, Young AJ (2004) Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch Biochem Biophys 430:37–48
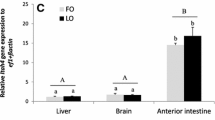
Elizondo-Reyna E, Medina-González R, Nieto-López MG, Ortiz-López R, Elizondo-González R, Powell MS, Ricque-Marie D, Cruz-Suárez LE (2016) Consumption of Ulva clathrata as a dietary supplement stimulates immune and lipid metabolism genes in Pacific white shrimp Litopenaeus vannamei. J Appl Phycol 28:3667–3677
Godard M, Décordé K, Ventura E, Soteras G, Baccou JC, Cristol JP, Rouanet JM (2009) Polysaccharides from the green alga Ulva rigida improve the antioxidant status and prevent fatty streak lesions in the high cholesterol fed hamster, an animal model of nutritionally-induced atherosclerosis. Food Chem 115:176–180
Gomez-Chang E, Larrea F, Martinez-Montes F (2012) Vías de señalización asociadas a la esteroidogénesis. Revista Especializada En Ciencias Quimico-Biológicas 15:24–36
Harrison KE (1990) The role of nutrition in maturation, reproduction, and embryonic development of decapod crustaceans: a review. J Shellfish Res 9:1–28
Hoa ND, Wouters R, Wille M, Thanh V, Dong TK, Van Hao N, Sorgeloos P (2009) A fresh-food maturation diet with an adequate HUFA composition for broodstock nutrition studies in black tiger shrimp Penaeus monodon (Fabricius, 1798). Aquaculture 297:116–121
Huang JH, Jiang SG, Lin HZ, Zhou FL, Ye L (2008) Effects of dietary highly unsaturated fatty acids and astaxanthin on the fecundity and lipid content of pond-reared Penaeus monodon (Fabricius) broodstock. Aquac Res 9:240–251
Johnson AL, Tilly JL, Levorse JM (1991) Possible role for arachidonic acid in the control of steroidogenesis in hen theca. Biol Reprod 44:338–344
Kacem M, Sellami M, Kammoun W, Frikha F, Miled N, Ben Rebah F (2011) Seasonal variations in proximate and fatty acid composition of viscera of Sardinella aurita, Sarpa salpa, and Sepia officinalis from Tunisia. J Aquat Food Prod Technol 20:233–246
Kangpanich C, Pratoomyot J, Siranonthana N, Senanan W (2016) Effects of arachidonic acid supplementation in maturation diet on female reproductive performance and larval quality of giant river prawn (Macrobrachium rosenbergii). PeerJ 4:e2735
Lampi AM, Dimberg LH, Kamal-Eldin A (1999) A study on the influence of fucosterol on thermal polymerisation of purified high oleic sunflower triacylglycerols. J Sci Food Agric 79:573–579
Lee S, Lee YS, Jung SH, Kang SS, Shin KH (2003) Anti-oxidant activities of fucosterol from the marine algae Pelvetia siliquosa. Arch Pharm Res 26:719–722
Liñan-Cabello MA, Medina-Zendejas R, Sanchez-Barajas M, Mena-Herrera A (2004) Effects of carotenoids and retinol in oocyte maturation of crayfish Cherax quadrucarinatus. Aquac Res 35:905–911
Lopes D, Moreira ASP, Rey F, da Costa E, Melo T, Maciel E, Rego A, Abreu MH, Domingues P, Calado R, Lillebø AI, Rosário Domingues M (2019) Lipidomic signature of the green macroalgae Ulva rigida farmed in a sustainable integrated multi-trophic aquaculture. J Appl Phycol 31:1369–1381
Maqsood S, Benjakul S (2010) Comparative studies of four different phenolic compounds on in vitro antioxidative activity and the preventive effect on lipid oxidation of fish oil emulsion and fish mince. Food Chem 119:123–132
Mirheydari SM, Emadi AM (2014) Influence of dietary HUFA on growth, survival, fecundity, egg diameter and fatty acid composition of broodstock white leg shrimp (Litopenaeus vannamei) tissues. World J Fish Mar Sci 6:340–349
Norberg B, Kleppe L, Andersson E, Thorsen A, Rosenlund G, Hamre K (2017) Effects of dietary arachidonic acid on the reproductive physiology of female Atlantic cod (Gadus morhua L.). Gen Comp Endocrinol 250:21–35
Palacios E, Racotta IS, Heras H, Marty Y, Moal J, Samain JF (2001) The relation between lipid and fatty acid composition of eggs and larval survival in white pacific shrimp (Penaeus vannamei, Boone, 1931). Aquac Int 96:531–543
Palacios E, Racotta IS, Arjona O, Marty Y, Le Coz JR, Moal J (2007) Lipid composition of the pacific lion-paw scallop, Nodipecten subnodosus, in relation to gametogenesis 2. Lipid classes and sterols. Aquaculture 266:266–273
Perez-Velazquez M, Lawrence AL, Gatlin DM, González-Félix ML, Bray WA (2002) Replacement of fresh dietary components by a dry feed for successful maturation of male Litopenaeus vannamei (Boone) broodstock. Aquac Res 33:1901–1905
Quinitio ET, Parado-Estepa FD, Millamena OM, Biona H (1996) Reproductive performance of captive Penaeus monodon fed various sources of carotenoids. In: Santiago CB, Coloso RM, Millamena OM, Borlongan IG (eds) Feeds for Small-Scale Aquaculture. Proceedings of the National Seminar-Workshop on Fish Nutrition and Feeds: SEAFDEC Aquaculture Department, Iloilo, pp 74–82
Regunathan C, Wesley SG (2006) Pigment deficiency correction in shrimp broodstock using Spirulina as a carotenoid source. Aquac Nutr 12:425–563
Rice-Evans C, Miller N, Paganga G (1997) Antioxidant properties of phenolic compounds. Trends Plant Sci 2:152–159
Rowley AF, Vogan CL, Taylor GW, Clare AS (2005) Prostaglandins in non-insectan invertebrates: recent insights and unsolved problems. J Exp Biol 208:3–14
Serviere-Zaragoza E, Hurtado MA, Manzano-Sarabia M, Mazariegos-Villareal A, Reza M, Arjona O, Palacios E (2014) Seasonal and interannual variation of fatty acids in macrophytes form the Pacific coast of Baja California Peninsula (Mexico). J Appl Phycol 27:1297–1306
Siddhanta AK, Goswami AM, Shanmugam M, Mody KH, Ramavat BK (2002) Sterols from marine green algae of Indian waters. J Indian Chem Soc 79:294–297
Sumpownon C, Engsusophon A, Siangcham T, Sugiyama E, Soonklang N, Meeratana P, Sobhon P (2015) Variation of prostaglandin E2 concentrations in ovaries and its effects on ovarian maturation and oocyte proliferation in the giant fresh water prawn, Macrobrachium rosenbergii. Gen Comp Endocrinol 23:129–138
Tsukimura B (2001) Crustacean vitellogenesis: its role in oocyte development. Am Zool 41:465–476
Wang L, Chen L, Qin J, Li E, Yu N, Du Z, Qi J (2015) Effect of dietary lipids and vitamin E on growth performance, body composition, anti-oxidative ability and resistance to Aromonas hydrophila challenge of juvenile Chinese mitten crab Eriocheir sinensis. Aquac Res 46:2544–2558
Wellburn AR (1994) The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J Plant Physiol 144:307–313
Wouters R, Lavens P, Nieto J, Sorgeloos P (2001) Penaeid shrimp broodstock nutrition: an updated review on research and development. Aquaculture 202:1–21
Xu H, Zhang Y, Luo K, Meng X, Luan S, Cao B, Kong J (2017) Arachidonic acid in diets for early maturation stages enhances the final reproductive performances of Pacific white shrimp (Litopenaeus vannamei). Aquaculture 479:556–563
Yano I (2000) Endocrine control of reproductive maturation in penaeid shrimp. In: Fingerman M, Nagabhushanam R (eds) Recent advances in marine biotechnology 4, Aquaculture. Pt. A. Enfield, NH: Science Publishers Inc, Enfield, pp 161–176
Yildiz G, Celikler S, Vatan O, Dere S (2012) Determination of the antioxidative capacity and bioactive compounds in green seaweed Ulva rigida C. Agardh. Int J Food Prop 15:1182–1189
Acknowledgements
The authors thank the owners and the technical team of the commercial hatchery (Larvas GranMar S.A. de C.V.), without which this work would not have been possible. The authors also thank Alberto Peña PhD and the Aonori Aquafarm directives for the donation of Ulva clathrata samples. DCCR received a doctorate scholarship from CONACYT, Mexico Grant No. 455811.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Corral-Rosales, C., Ricque-Marie, D., Cruz-Suárez, L.E. et al. Fatty acids, sterols, phenolic compounds, and carotenoid changes in response to dietary inclusion of Ulva clathrata in shrimp Litopenaeus vannamei broodstock. J Appl Phycol 31, 4009–4020 (2019). https://doi.org/10.1007/s10811-019-01829-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-01829-2