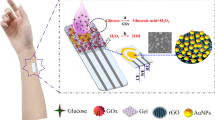
Abstract
Herein, a self-powered lactate biosensor (SPLBs) was assembled to detect lactate in sweat based on flexible textile matrix enzymatic electrodes. A porous three-dimensional electrode with high flexibility and electrical conductivity was obtained by decorating composites of reduced graphene oxide and carboxylate multiwalled carbon nanotubes onto a cellulose fabric substrate. The excellent enzyme embedding method using a gel electrolyte showed a Michaelis–Menten constant of 1.46 mM, indicating the high enzymatic activity of lactate dehydrogenase. Moreover, the assembled (SPLBs) displayed a sensitivity of 3.16 µW mM−1 cm−2 with a linear range of 0–10 mM and a detection limit of 9.49 µM. Additionally, the biosensor has good tensile flexural stability, selectivity, and long-term stability. When integrated into clothing, the (SPLBs) recovered 91.5–102.3% of lactate from real sweat, with a relative standard deviation of less than 4.16%. This biosensor is promising for sensing lactate in human sweat.
Graphical abstract









Similar content being viewed by others
References
Cheng Y, Wang K, Xu H, Li T, Jin Q, Cui D (2021) Recent developments in sensors for wearable device applications. Anal Bioanal Chem 413:6037–6057. https://doi.org/10.1007/s00216-021-03602-2
Huang X et al (2022) Epidermal self-powered sweat sensors for glucose and lactate monitoring. Bio-Design and Manufacturing 5(1):201–209. https://doi.org/10.1007/s42242-021-00156-1
Wang F, Cai R, Tan W (2023) Self-powered biosensor for a highly efficient and ultrasensitive dual-biomarker assay. Anal Chem 95(14):6046–6052. https://doi.org/10.1021/acs.analchem.3c00097
Bollella P, Boeva Z, Latonen R-M, Kano K, Gorton L, Bobacka J (2021) Highly sensitive and stable fructose self-powered biosensor based on a self-charging biosupercapacitor. Biosens Bioelectron 176:112909. https://doi.org/10.1016/j.bios.2020.112909
Han O, Jiang D, Fan Y, Wang ZL, Li Z (2021) Self-powered technology for next-generation biosensor. Sci Bull 66:1709–1712. https://doi.org/10.1016/j.scib.2021.04.035
Tong C, Liu H, Mo Y, Li J, Liu X, Pang L (2023) In-situ growth of enzyme/copper phosphate hybrids on carbon cloth surface as self-powered electrochemical glucose biosensor. Biochem Eng J 193:108860. https://doi.org/10.1016/j.bej.2023.108860
Yi J, Xianyu Y (2022) Gold nanomaterials-implemented wearable sensors for healthcare applications. Adv Funct Mater 32(19):2113012. https://doi.org/10.1002/adfm.202113012
Promphet N, Ummartyotin S, Ngeontae W, Puthongkham P, Rodthongkum N (2021) Non-invasive wearable chemical sensors in real-life applications. Analytica Chimica Acta 1179:338643. https://doi.org/10.1016/j.aca.2021.338643
Jiang Y et al (2023) Hemocytes in blue mussel Mytilus edulis adopt different energy supply modes to cope with different BDE-47 exposures,. Sci Total Environ 885:163766. https://doi.org/10.1016/j.scitotenv.2023.163766
Schuck A, Kim HE, Moreira JK, Lora PS, Kim Y-S (2021) A graphene-based enzymatic biosensor using a common-gate field-effect transistor for l-lactic acid detection in blood plasma samples. Sensors 21(5):1852. https://doi.org/10.3390/s21051852
A BG, C CC, G LR, D OA, A AJ (2022) Tracing the lactate shuttle to the mitochondrial reticulum. Exp Mol Med 54:1–16. https://doi.org/10.1038/s12276-022-00802-3
Zhukova GV, Sutormin OS, Sukovataya IE, Maznyak NV, Kratasyuk VA (2023) Bioluminescent-triple-enzyme-based biosensor with lactate dehydrogenase for non-invasive training load monitoring. Sensors 23(5):2865. https://doi.org/10.3390/s23052865
Golveia JCS, Bara MTF, Santiago MF, Campos LC, Schimidt F (2020) Cocoa Agro-Industrial Residue (Theobroma cacao) as Inducer of the Production of Fungal Laccase and Kojic Acid for Application in the Biodegradation of 17-α-Ethinylestradiol,. J Braz Chem Soc 31(10):2023–2029. https://doi.org/10.21577/0103-5053.20200102
Higashi M, Toyodome T, Kano K, Amao Y (2023) Photoelectrochemical lactate production from pyruvate via in situ NADH regeneration over a hybrid system of CdS photoanode and lactate dehydrogenase,. Electrochim Acta 460:142590. https://doi.org/10.1016/j.electacta.2023.142590
Shortall K et al (2023) Coupled immobilized bi-enzymatic flow reactor employing cofactor regeneration of NAD(+) using a thermophilic aldehyde dehydrogenase and lactate dehydrogenase. Green Chem 25(11):4553–4564. https://doi.org/10.1039/d3gc01536j
Zhang J et al (2021) Self-healing mechanism and conductivity of the hydrogel flexible sensors: a review. Gels 7(4):216
Song Z et al (2023) Flexible and wearable biosensors for monitoring health conditions. Biosens-Basel 13(6):630. https://doi.org/10.3390/bios13060630
Ma J et al (2022) Oil-water self-assembly engineering of Prussian blue/quantum dots decorated graphene film for wearable textile biosensors and photoelectronic unit. Chem Eng J 427:131824. https://doi.org/10.1016/j.cej.2021.131824
Wang R, Zhai Q, An T, Gong S, Cheng W (2021) Stretchable gold fiber-based wearable textile electrochemical biosensor for lactate monitoring in sweat. Talanta 222:121484. https://doi.org/10.1016/j.talanta.2020.121484
Qin H, Wang Z, Yu Q, Xu Q, Hu X-Y (2022) Flexible dibutyl phthalate aptasensor based on self-powered CNTs-rGO enzymatic biofuel cells,. Sens Actuators B-Chem 371:13246. https://doi.org/10.1016/j.snb.2022.132468
Jiang D et al (2022) In-situ preparation of lactate-sensing membrane for the noninvasive and wearable analysis of sweat,. Biosens Bioelectron 210:114303. https://doi.org/10.1016/j.bios.2022.114303
Yang Y, Su Y, Zhu X, Ye D, Chen R, Liao Q (2022) Flexible enzymatic biofuel cell based on 1, 4-naphthoquinone/MWCNT-Modified bio-anode and polyvinyl alcohol hydrogel electrolyte,. Biosens Bioelectron 198:113833. https://doi.org/10.1016/j.bios.2021.113833
Fan S et al (2022) Stretchable and bendable textile matrix based on cellulose fibers for wearable self-powered glucose biosensors. Cellulose 29(16):8919–8935. https://doi.org/10.1007/s10570-022-04820-2
Paik JJ, Jang B, Nam S, Guo LJ (2023) A Transparent Poly(vinyl alcohol) Ion-Conducting Organohydrogel for Skin-Based Strain-Sensing Applications. Adv Healthc Mater 12:2300076. https://doi.org/10.1002/adhm.202300076
Zhang J et al (2023) High-Energy-Density Zinc-Air Microbatteries with Lean PVA-KOH-K2CO3 Gel Electrolytes,. Acs Appl Mater Interfaces 15(5):6807–6816. https://doi.org/10.1021/acsami.2c19970
Rahmani S, Olad A, Rahmani Z (2023) Preparation of self-healable nanocomposite hydrogel based on Gum Arabic/gelatin and graphene oxide: study of drug delivery behavior. Polym Bull 80(4):4117–4138. https://doi.org/10.1007/s00289-022-04247-6
Arularasu MV, Sendhil M, Rajendran TV, Mani G, Aljuwayid AM, Habila MA (2022) Recent advantages of zinc oxide/carbon nanotubes/reduced graphene oxide based nanocomposite for the visible light photodegradation. Inorg Chem Commun 139:109332. https://doi.org/10.1016/j.inoche.2022.109332
Teimuri-Mofrad R, Hadi R, Abbasi H (2019) Synthesis and characterization of ferrocene-functionalized reduced graphene oxide nanocomposite as a supercapacitor electrode material. J Organomet Chem 880:355–362
da Silva AP, do Amaral Montanheiro TL, Montagna LS, Andrade PF, Duran N, Lemes AP (2019) Effect of carbon nanotubes on the biodegradability of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) nanocomposites,. J Appl Polym Sci 136:48020. https://doi.org/10.1002/app.48020
Rajaitha PM, Shamsa K, Sheebha I, Vidhya B, Maheskumar V, Rajesh S (2019) Influence of the positioning of the incorporated carbon nanostructures on the morphology and photocatalytic activity of microwave synthesized ZnO nanorods. J Nanosci Nanotechnol 19(8):5303–5309. https://doi.org/10.1166/jnn.2019.16836
Alagumalai K et al (2022) A portable advanced electrocatalyst for polyphenolic chlorogenic acid evaluation in food samples. Chem Eng J 435:134796. https://doi.org/10.1016/j.cej.2022.134796
Wang H, Xie Y (2022) Hydrogen bond enforced polyaniline grown on activated carbon fibers substrate for wearable bracelet supercapacitor. J Energy Storage 52:105042. https://doi.org/10.1016/j.est.2022.105042
Zafar MN, Aslam I, Ludwig R, Xu G, Gorton L (2019) An efficient and versatile membraneless bioanode for biofuel cells based on Corynascus thermophilus cellobiose dehydrogenase,. Electrochim Acta 295:316–324. https://doi.org/10.1016/j.electacta.2018.10.047
Sun H et al (2023) Live-cell imaging reveals redox metabolic reprogramming during zygotic genome activation. J Cell Physiol 238(9):2039–2049. https://doi.org/10.1002/jcp.31054
Shen F et al (2019) Two-dimensional graphene paper supported flexible enzymatic fuel cells. Nanoscale Adv 1(7):2562–2570. https://doi.org/10.1039/c9na00178f
Wang L-L, Shao H-H, Wang W-J, Zhang J-R, Zhu J-J (2018) Nitrogen-doped hollow carbon nanospheres for high-energy-density biofuel cells and self-powered sensing of microRNA-21 and microRNA-141. Nano Energy 44:95–102. https://doi.org/10.1016/j.nanoen.2017.11.055
Chelliah M, Nesakumar N, Thandavan K, Sethuraman S, Krishnan UM, Rayappan JBB (2014) An Electrochemical Biosensor with Nano-Interface for Lactate Detection Based on Lactate Dehydrogenase Immobilized on Iron Oxide Nanoparticles. Nanosci Nanatechnol Lett 6(3):242–249. https://doi.org/10.1166/nnl.2014.1744
Iacovino LG et al (2022) Allosteric transitions of rabbit skeletal muscle lactate dehydrogenase induced by pH-dependent dissociation of the tetrameric enzyme. Biochim 199:23–35. https://doi.org/10.1016/j.biochi.2022.03.008
Jeyabarathi P, Rajendran L, Lyons MEG (2022) Reaction-diffusion in a packed-bed reactors: Enzymatic isomerization with Michaelis-Menten Kinetics. J Electroanal Chem 910:116184. https://doi.org/10.1016/j.jelechem.2022.116184
Hui Y, Wang H, Zuo W, Ma X (2022) Spider nest shaped multi-scale three-dimensional enzymatic electrodes for glucose/oxygen biofuel cells,. Int J Hydrogen Energy 47(9):6187–6199. https://doi.org/10.1016/j.ijhydene.2021.11.2100360-3199
Hu T et al (2021) A pH-responsive ultrathin Cu-based nanoplatform for specific photothermal and chemodynamic synergistic therapy,. Chem Sci 12(7):2594–2603. https://doi.org/10.1039/d0sc06742c
Hui Y, Ma X, Qu F (2019) Flexible glucose/oxygen enzymatic biofuel cells based on three-dimensional gold-coated nickel foam. J Solid State Electrochem 23(1):169–178. https://doi.org/10.1007/s10008-018-4099-4
Wan J, Mi L, Tian Z, Li Q, Liu S (2020) A single-liquid miniature biofuel cell with boosting power density via gas diffusion bioelectrodes,. J Mater Chem B 8(16):3550–3556. https://doi.org/10.1039/c9tb02100k
Yang L et al (2022) A novel strategy for the detection of pyruvate in fermentation processes based on well-distributed enzyme-inorganic hybrid nanoflowers on thiol graphene modified gold electrodes,. Electrochim Acta 427:140855. https://doi.org/10.1016/j.electacta.2022.140855
Liu H et al (2023) A peptide-target-aptamer electrochemical biosensor for norovirus detection using a black phosphorous nanosheet@Ti3C2-Mxene nanohybrid and magnetic covalent organic framework. Talanta 258:124433. https://doi.org/10.1016/j.talanta.2023.124433
Duong HD, Rhee JI (2021) Ratiometric fluorescent biosensors for glucose and lactate using an oxygen-sensing membrane. Biosensors-Basel 11(7):208. https://doi.org/10.3390/bios11070208
Fitriana M, Hiraka K, Ikebukuro K, Sode K, Tsugawa W (2022) A Thiol-reactive Phenazine Ethosulfate-A Novel Redox Mediator for Quasi-direct Electron-transfer-type Sensors. Sens Mater 34(6):2105–2124
Liu M, Yang M, Wang M, Wang H, Cheng J (2022) A flexible dual-analyte electrochemical biosensor for salivary glucose and lactate detection. Biosensors 12(4):210. https://doi.org/10.3390/bios12040210
Payne ME, Zamarayeva A, Pister VI, Yamamoto NAD, Arias AC (2019) Yamamoto NAD, Arias AC “Printed, Flexible Lactate Sensors: Design Considerations Before Performing On-Body Measurements. Scientific reports 9:13720. https://doi.org/10.1038/s41598-019-49689-7
Maduraiveeran G, Chen A (2021) Design of an enzyme-mimicking NiO@ Au nanocomposite for the sensitive electrochemical detection of lactic acid in human serum and urine. Electrochimica Acta 368:137612. https://doi.org/10.1016/j.electacta.2020.137612
Acknowledgements
This work was supported by the Ph.D. Research Initiation Fund of Xi’an Polytechnic University (No: 310/107020509 and No: 310/107020555); Natural Science Basic Research Program of Shaanxi (Program No. 2021JQ-663, 2023-JC-QN-0565 and No. 2021JQ-671); and The Scientific Research Program Funded by Shaanxi Provincial Education Department (Program No. 21JK0662) and National Natural Science Foundation of China (NO. 22208256). The authors declare that they had no conflict of interest.
Author information
Authors and Affiliations
Contributions
ZX, WZ, and YH were mainly responsible for the experiments and writing of the article. HL, LQ, and RM were responsible for the drawing of the article. QW, HL, and HW were responsible for the revisions and details of the article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xia, Z., Zuo, W., Li, H. et al. Wearable cellulose textile matrix self-powered biosensor sensing lactate in human sweat. J Appl Electrochem 54, 1137–1152 (2024). https://doi.org/10.1007/s10800-023-02010-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-023-02010-x