Abstract
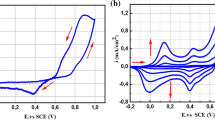
A non-enzymatic glucose sensor using a nickel particles/polyaniline composite has been synthesized on an indium tin oxide electrode. The PAni thin films were deposited onto the ITO surfaces using a repeated potential cycling technique in an aqueous solution containing aniline, sulfuric acid, and lithium perchlorate. Nickel particles were incorporated into the PAni/ITO surfaces using chronopotentiometry. Scanning electron micrograph and X-ray diffraction were employed to investigate the surface morphology and structure of the Ni-PAni composite, while Ultraviolet–visible spectroscopy was used to study the optical properties. The modified electrode was electrochemically characterized using cyclic voltammetry and impedance spectroscopy. The effect of PAni thin film thickness on the nickel deposition process has also been studied. Nickel was chosen due to its reduction potential being within the range where the PAni layer is in a reduced, non-conducting state. The electroactivity of the Ni-PAni/ITO electrode was evaluated through cyclic voltammetry and chronoamperometry and explored its potential for electrocatalytic glucose oxidation in an alkaline (NaOH) electrolyte. Excellent linearity in the peak oxidation current of glucose within the concentration range from 0.02 mM to 9 mM was observed with a high linear regression coefficient of 0.997. The Ni-PAni/ITO electrode displayed a high sensitivity of 215.8 mA mM−1 cm−2 in addition to the fast response time, which is less than 2 s. These results suggest that the Ni-PAni composite has the potential to be an effective electrode material to develop a cost-effective glucose sensor.
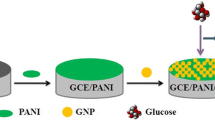
Graphical abstract
Schematic illustration of the preparation of Ni-polyaniline electrode for glucose sensing

Research Highlights
-
Nickel nanoparticles were incorporated in the polyaniline thin films by chronopotentiometry method.
-
The prepared Ni-polyaniline hybrids materials exhibit high sensitivity of 215.8 mA mM−1 cm−2
-
low-response time (2 s), good linearity in the concentration range from 0.1 mM to 12 mM, and low detection limit (0.01mM, S/N = 3).
-
The good analytical performance, low cost, and facile fabrication method make this new electrode material promising for the development of effective glucose sensors.














Similar content being viewed by others
References
Qiao WM, Zhao Y, Liang H, Li J, Luo TS, Lu YS, Shi S, Lu X, Sun W, Ping X (2020) Electrochemical non-enzymatic glucose sensors: recent progress and perspectives. Chem Commun. https://doi.org/10.1039/d0cc05650b
Vernekar PR, Purohit B, Shetti NP, Chandra P (2021) Glucose modified carbon paste sensor in the presence of cationic surfactant for mefenamic acid detection in urine and pharmaceutical samples. Microchemistry. https://doi.org/10.1016/j.microc.2020.105599
Rostami-avanroudi S, Babakhanian A (2021) New electrochemical sensor for direct quantification of vitamin K in human blood serum. Microchem J. https://doi.org/10.1016/j.microc.2020.105716
Ghanbary E, Asiabani Z, Hosseini N, Kiaie SH, Kaki S, Ghasempour H, Babakhanian A (2020) The development of a new modified graphite pencil electrode for quantitative detection of Gibberellic acid (GA3) herbal hormone. Microchem J. https://doi.org/10.1016/j.microc.2020.105005
Azizi Z, Babakhanian (2018) A fabricating a new electrochemically modified pencil graphite electrode based on acetophenone (2, 4-dinitrophenyl) hydrazone for determining selenium in food and water samples. Anal Methods. https://doi.org/10.1039/C8AY01959B
Schierenbeck F, Franco-Cereceda A, Liska J (2017) Accuracy of 2 different continuous glucose monitoring systems in patients undergoing cardiac surgery: intravascular microdialysis versus subcutaneous tissue monitoring. J Diabetes Sci Technol. https://doi.org/10.1177/1932296816651632
Zhang L, Wang J, Liu F, Xiong Y, Liu Z, Jiang D, Li Y, Tu D, Wang Y, Pu X (2017) Rapid detection of blaNDM-1 in multidrug-resistant organisms using a novel electrochemical biosensor. RSC Adv. https://doi.org/10.1039/C6RA27916C
Wei M, Qiao Y, Zhao H, Liang J, Li T, Luo Y, Lu S, Shi X, Lu W, Sun X (2021) Electrochemical non-enzymatic glucose sensors: recent progress and perspectives. Chem Commun. https://doi.org/10.1039/D0CC05650B
Delk S, Parsanta ASMD, Pashupulla AP, Srivastava KA, Yadav SK, Saxena S (2022) Enzyme based glucose biosensor—an overview. J Pharm Negat Results. https://doi.org/10.47750/pnr.2022.13.S09.1090
Radhakrishnan S, Lakshmy S, Santhosh S, Kalarikkal N, Chakraborty B, Rout CS (2022) Recent developments and future perspective on electrochemical glucose sensors based on 2D materials. Biosensors. https://doi.org/10.3390/bios12070467
Babulal SM, Chen SM, Palani R, Venkatesh K, Haidyrah AS, Ramaraj SK, Yang CC, Karuppiah C (2021) Graphene oxide template based synthesis of NiCO2O4 nanosheets for high performance non-enzymatic glucose sensor. Colloids Surf A. https://doi.org/10.1016/J.COLSURFA.2021.126600
Wang F, Chen X, Chen L, Yang J, Wang Q (2019) High-performance non-enzymatic glucose sensor by hierarchical flower-like nickel(II)-based MOF/carbon nanotubes composite. Mater Sci Eng C. https://doi.org/10.1016/j.msec.2018.11.004
Sadeghi E, Rahimi F, Azizi Z, Kaki S, Babakhanian A (2023) Fabrication of a sensitive electrochemical sensor based on hybrid polyamide/chromotropic acid nanofibers electrospun on glassy carbon electrode for Hg2+ sensing in drinking water and canned fish samples. Food Chem. https://doi.org/10.1016/j.foodchem.2023.135467
Ngun MY, MohamadNor N, Ramli NH, AbdulRazak K (2022) Platinum nanoparticles modified electrode for glucose sensor. Mater Today Proc. https://doi.org/10.1016/j.matpr.2022.06.569
Shabbir SA, Tariq S, Gbahar Ashiq M, Khan WA (2019) Non-enzymatic glucose sensor with electrodeposited silver/carbon nanotubes composite electrode.Biosci Rep. 10.1042/BSR20181983
Müsse A, La Malfa F, Brunetti V, Rizzi F, De Vittorio M (2021) Flexible enzymatic glucose electrochemical sensor based on polystyrene-gold electrodes. Micromachines. https://doi.org/10.3390/mi12070805
Chalil Oglou R, Ulusoy Globoid TG, Ozbay E, Karadas F (2021) Electrodeposited cobalt hexacyanoferrate electrode as a non-enzymatic glucose sensor under neutral conditions. Anal Chim Acta. https://doi.org/10.1016/j.aca.2021.339188
Chong SF, Razak KA, Nor NM, Ridhuan NS, Zakaria ND (2019) Electrochemical glucose detection using screen-printed carbon electrode modified silica-encapsulated iron oxide nanoparticles. Mater Today Proc. https://doi.org/10.1016/j.matpr.2019.06.562
Phetsang S, Kidkhunthod P, Chanlek N, Jakmunee J, Mungkornasawakul P, Ounnunkad K (2021) Copper/reduced graphene oxide flm modified electrode for non-enzymatic glucose sensing application. Sci Rep 1:10. https://doi.org/10.1038/s41598-021-88747-x
OsunaV V-R, Zaragoza-Contreras EA, Estrada-MorenoIA DRB (2022) Progress of polyaniline glucose sensors for diabetes mellitus management utilizing enzymatic and non-enzymatic detection. Biosensors. https://doi.org/10.3390/bios12030137
Abolghasemi MM, Ghorbani-Cheghamarania A, Babakhanian A (2017) A novel electrochemical sensing platform based on Pt/PPy/Eosin-Y for the determination of cadmium. New J Chem. https://doi.org/10.1039/C7NJ02080E
Belgherbi O, Chouder D, Lakhdari D, Dehchar C, Laidoudi S, Lamiri L, Hamam A, Seid L (2020) Correction to: Enzyme-free glucose sensor based on star-like copper particles-polyaniline composite film. J Inorg Organomet Polym Mater. https://doi.org/10.1007/s10904-020-01609-3
Lamiri L, BelgherbiO DC, Laidoudi S, Tounsi A, Nessark B, Habelhames F, Hamam A, Gourari B (2020) Performance of polybithiophene-palladium particles modified electrode for non-enzymatic glucose detection. Synth Met. https://doi.org/10.1016/j.synthmet.2020.116437
Belgherbi O, Seid L, Lakhdari D, Chouder D, Akhtar MS, Saeed M (2021) Optical and morphological properties of electropolymerized semiconductor polyaniline thin films: effect of thickness. J Electron Mater. https://doi.org/10.1007/s11664-021-08896-7
Korent A, Žagar Soderžnik K, Šturm S, Žužek Rožman K (2020) A correlative study of polyaniline electropolymerization and its electrochromic behavior. J Electrochem Soc. https://doi.org/10.1149/1945-7111/ab9929
Fuseini M, Yousry Zaghloul MM, Elkady MF, El-Shazly AH (2022) Evaluation of synthesized polyaniline nanofibres as corrosion protection film coating on copper substrate by electrophoretic deposition. J Mater Sci. https://doi.org/10.1007/s10853-022-06994-3
Goswami M, Ghosh R, Meikap AK (2016) Synthesis and characterization of new polyaniline-Ni nanocomposite. J Comput Theor Nanosci. https://doi.org/10.1166/asl.2016.6812
Belgherbi O, Chouder D, Saeed MA (2018) Elaboration and characterization of ITO electrode modified by transition metal dispersed into polyaniline thin films. Optics. https://doi.org/10.1016/j.ijleo.2018.06.102
Mahbubur Rahman Md, Saleh Ahammad AJ, Jin JH, Ah SJ, Lee JJ (2010) A comprehensive review of glucose biosensors based on nanostructured metal-oxides. Sens. https://doi.org/10.3390/s100504855
Lopes LC, Santos A, Bueno PR (2022) An outlook on electrochemical approaches for molecular diagnostics assays and discussions on the limitations of miniaturized technologies for point-of-care devices. Sens Actuators Rep. https://doi.org/10.1016/j.snr.2022.100087
Niu X, Lan M, Zhao H, Chen C (2013) Highly sensitive and selective nonenzymatic detection of glucose using three-dimensional porous nickel nanostructures. Anal Chem. https://doi.org/10.1021/ac3030976
Bezza A, Ouennoughi Y, Bouzerafa B, Aggoun D, Bezzi H, López D, Fernández García M, Ourari A (2018) New quaternized poly(4-vinylpyridine-co-divinylbenzene) material containing nickel(II) Schiff base complex:synthesis, thermogravimetry, and application or heterogeneous electrooxidation of ethanol. Res Chem Intermed. https://doi.org/10.1007/s11164-018-3524-8
Lu LM, Zhang L, Qu FL, Lu HX, Zhang XB (2009) A nano-Ni based ultrasensitive nonenzymaticelectrochemical sensor for glucose: enhancing sensitivity through a nanowire array strategy. Biosens Bioelectron. https://doi.org/10.1016/j.bios.2009.06.041
Jafarian M, Forouzandeh F, Danaee I, Mahjani MG (2009) Electrocatalytic oxidation of glucose on Ni and NiCu alloy modified glassy carbon electrode. J Solid State Electrochem. https://doi.org/10.1007/s10008-008-0632-1
Dehchar C, Chikouche I, Kherrat R, Zougar S, Zouaoui A (2017) Electrocatalytic oxidation of ascorbic acid at polypyrrole thin film incorporating palladium particles. Surf Rev Lett. https://doi.org/10.1142/S0218625X17501189
Zhang Y, Xu F, Sun Y, Shi Y, Wen Z, Li Z (2011) Assembly of Ni (OH)2 nanoplates on reduced graphene oxide: a two dimensional nanocomposite for enzyme-free glucose sensing. J Mater Chem. https://doi.org/10.1039/C1JM11641J
Zhu X, Jiao Q, Zhang C, Zuo X, Xiao X, Liang Y, Nan J (2013) Amperometric nonenzymatic determination of glucose based on a glassy carbon electrode modified with nickel (II) oxides and graphene. Microchim Acta. https://doi.org/10.1007/s00604-013-0955-1
Milakin KA, Korovin AN, Moroz EV, Levon K, Guiseppi-Elie A, Sergeyev VG (2013) Polyaniline-based sensor material for potentiometric determination of ascorbic acid. Electroanalysis. https://doi.org/10.1002/elan.201300023
Sun A, Zheng J, Sheng Q (2012) A highly sensitive non-enzymatic glucose sensor based on nickel and multi-walled carbon nanotubes nanohybrid films fabricated by one-step co-electrodeposition in ionicliquids. Electrochim Acta. https://doi.org/10.1016/j.electacta.2012.01.007
Author information
Authors and Affiliations
Contributions
All authors reviewed the manuscript."
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Belgherbi, O., Messoudi, M., Bezi, H. et al. Incorporation of nickel particles into a polyaniline thin film for non-enzymatic glucose sensing in alkaline medium. J Appl Electrochem 54, 851–863 (2024). https://doi.org/10.1007/s10800-023-01979-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-023-01979-9