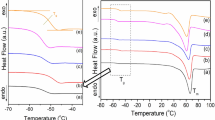
Abstract
The effect of the lithium salt LiTFSI molality into the PEO-based electrolyte on the ionic conductivity was investigated in this work. To begin, the experimental evolution of LiTFSI-PEO electrolyte's ionic conductivity as a function of molality was analyzed, and hypotheses were put out to explain how ionic transport occurs in a heterogeneous microstructure of polymer electrolytes. To forecast the ionic conductivity in the electrolyte LiTFSI-PEO as a function of molality, an empirical mathematical model was then proposed, taking the phenomena predicted to occur into account. The proposed theoretical model presupposes that lithium salt's solvation state (total or partial solvation) and the steric effect brought on by heterogeneous areas are related to the conduction of ions (at the microscopic scale). The model is adjusted to accurately depict the entire experimental curve, which is thought to have three distinct domains. In the first domain, where the molality m is less than 1 mol/kg, the oxygen atoms completely solvate the lithium salt. The conduction is believed to be favorable in this situation since the released solvated lithium ions linearly fluctuate against the molality. The partial solvation of lithium corresponds to the second domain (1 mol/kg ≾ m ≾ 2 mol/kg), where the conductivity of the polymer electrolyte slightly increases with increasing molality. In the third domain, where m is greater than 2 mol/kg, the loss in ionic conductivity is caused by steric effects, as some of the lithium salt (LiTFSI) does not ionize and becomes immobilized, obstructing the transport pathway. Finally, the model was expanded to include how temperature affects ionic conductivity. The prediction model was effectively validated when it was put up against the findings of experiments conducted by various authors.
Graphical abstract








Similar content being viewed by others
Abbreviations
- \(\mathrm{c}=\frac{{\mathrm{c}}_{+}}{{\upnu }_{+}}=\frac{{\mathrm{c}}_{-}}{{\upnu }_{-}}\) :
-
Normalized molarity of binary salt, [\(\mathrm{mol}\ {\mathrm{m}}^{-3}\)]
- \({\mathrm{c}}_{0}\) :
-
Polymer molarity, [\(\mathrm{mol}\ {\mathrm{m}}^{-3}\)]
- \(\mathrm{D}\) :
-
Solute diffusion coefficient, [\({\mathrm{m}}^{2}/\mathrm{s}\)]
- \(\mathcal{D}\) :
-
Thermodynamic diffusion coefficient of solute, [\({\mathrm{m}}^{2}/\mathrm{s}\)]
- \({\mathcal{D}}_{\pm }\) :
-
Pairwise diffusion coefficient of cations with respect to anions, [\({\mathrm{m}}^{2}/\mathrm{s}\)]
- \({\mathcal{D}}_{0-}\) :
-
Pairwise diffusion coefficient of anions with respect to polymeric solvent, [\({\mathrm{m}}^{2}/\mathrm{s}\)]
- \(\mathrm{F}\) :
-
Faraday constant, [96 486 C/mol]
- \({\mathrm{f}}_{\pm }\) :
-
Mean molar activity coefficient \({\left({\mathrm{f}}_{+} {\mathrm{f}}_{-}\right)}^{0.5}\)
- \({\mathrm{f}}_{+}\) :
-
Molar activity coefficients of cations
- \({\mathrm{f}}_{-}\) :
-
Molar activity coefficients of anions
- \(\mathbf{i}\) :
-
Density of ionic current, [\(\mathrm{A}\ {\mathrm{m}}^{-2}\)]
- \(\mathbf{m}\) :
-
Molality of the lithium salt, [\(\mathrm{mol}\ {\mathrm{kg}}^{-1}\)]
- \({\mathbf{m}}^{*}\) :
-
Starting partial dissolution molality, [\(\mathrm{mol}\ {\mathrm{kg}}^{-1}\)]
- \({\mathbf{m}}_{\mathrm{lim}}\) :
-
Critical molality marking the starting \(\upepsilon\) point of the decrease of the ionic conductivity, [\(\mathrm{mol}\ {\mathrm{kg}}^{-1}\)]
- \({\mathrm{m}}_{\mathrm{PEO}}\) :
-
Mass of PEO, [\(\mathrm{kg}\)]
- \({\mathrm{m}}_{\mathrm{salt}}\) :
-
Mass of lithium salt, [\(\mathrm{kg}\)]
- \({\mathrm{M}}_{\mathrm{salt}}\) :
-
Molar mass of the salt, [\(\mathrm{g}\ {\mathrm{mol}}^{-1}\)]
- \({\mathrm{M}}_{\mathrm{EO}}\) :
-
Molar mass of the monomer ethylene oxide (EO), [\(\mathrm{g}\ {\mathrm{mol}}^{-1}\)]
- \({\mathrm{M}}_{\mathrm{PEO}}\) or \({\mathrm{M}}_{\mathrm{w}}\) :
-
Molar mass of the polymer ethylene oxide (PEO), [\(\mathrm{g}\ {\mathrm{mol}}^{-1}\)]
- \({\mathrm{n}}_{\mathrm{PEO}}\) :
-
Moles number of the PEO, [\(\mathrm{mol}\)]
- \({\mathrm{n}}_{\mathrm{salt}}\) :
-
Moles number of the lithium salt, [\(\mathrm{mol}\)]
- \(\mathrm{t}\) :
-
Time, [\(\mathrm{s}\)]
- \({\mathrm{t}}_{+}^{0}\) :
-
Transference number of the cation
- \(\left(1+\frac{\partial {\mathrm{lnf}}_{\pm }}{\partial \mathrm{lnc}}\right)\) :
-
Thermodynamic factor
- \({\mathbf{v}}_{0}\) :
-
Solvent velocity (here the polymer PEO i.e., polyethylene oxide), [\(\mathrm{m}\ {\mathrm{s}}^{-1}\)]
- \({\mathrm{V}}_{\mathrm{el}}\) :
-
Total volume of the electrolyte, [\(\mathrm{L}\)]
- \({\mathrm{V}}_{\mathrm{PEO}}\) :
-
Total volume occupied by PEO fibers, [\(\mathrm{L}\)]
- \({\mathrm{V}}_{\mathrm{salt}}\) :
-
Total volume occupied by the lithium salt, [\(\mathrm{L}\)]
- \({\overline{\mathrm{V}} }_{\mathrm{salt}}\) :
-
Partial molar volume of lithium salt, [\({\mathrm{m}}^{3}/\mathrm{mol}\)]
- \({\overline{\mathrm{V}} }_{\mathrm{PEO}}\) :
-
Partial molar volume of the PEO, [\({\mathrm{m}}^{3}/\mathrm{mol}\)]
- R:
-
Universal gas constant, [\(\mathrm{J}\ {\mathrm{mol}}^{-1}\, {\mathrm{K}}^{-1}\)]
- \(\mathrm{T}\) :
-
Temperature of the polarized electrochemical cell, [\(\mathrm{K}\)]
- \({\mathrm{T}}_{\mathrm{r}}\) :
-
Reference temperature at which model coefficients were deduced, \(\mathrm{K}\)
- \(\upepsilon\) :
-
Ratio of hard volume by electrolyte volume
- \(\upkappa\) :
-
Ionic conductivity, [\(\mathrm{S}{\, \mathrm{m}}^{-1}]\)
- \({\upkappa }_{\mathrm{eff}}\) :
-
Effective ionic conductivity, [\(\mathrm{S}{\, \mathrm{m}}^{-1}]\)
- \({\upkappa }_{\mathrm{Newman}}\) :
-
Ionic conductivity versus Newman theory, [\(\mathrm{S}{\, \mathrm{m}}^{-1}]\)
- \({\upkappa }_{\mathrm{model}}\) :
-
Ionic conductivity versus our model \({\upkappa }_{\mathrm{eff}}\), [\(\mathrm{S}{\, \mathrm{m}}^{-1}]\)
- \({\upnu }_{+}\) :
-
Stoichiometric coefficient of cations of the lithium salt
- \({\upnu }_{-}\) :
-
Stoichiometric coefficient of anions j of the lithium salt
- \(\upnu\) :
-
Number of moles of salt per unit of mole of electrolyte \({\upnu }_{+}+{\upnu }_{-}\)
- ρ:
-
Density of the electrolyte, [\(\mathrm{kg}\ {\mathrm{m}}^{-3}\)]
- \(\Phi\) :
-
Electric potential, [\(\mathrm{V}\)]
- \({\mathrm{\chi}}\) :
-
Fraction of solvated lithium ions
- \(\mathrm{Eff}\) :
-
Effective
- \(\mathrm{Exp}\) :
-
Experimental
- \(\mathrm{Newman}\) :
-
Based on Newman model
- \(\mathrm{Model}\) :
-
Modelling
- \(\mathrm{Salt}\) :
-
Lithium salt LiTFSI
- \(\mathrm{PEO}\) :
-
Poly (ethylene oxide) PEO
- \(+\) :
-
Cations
- \(-\) :
-
Anions
- \(0\) :
-
Polymeric solvent (PEO)
References
Mao G, Saboungi M-L, Price DL, Armand MB, Howells WS (2000) Structure of liquid PEO-LiTFSI electrolyte. Phys Rev Lett 84:5536–5539. https://doi.org/10.1103/PhysRevLett.84.5536
Nilsson V, Bernin D, Brandell D, Edström K, Johansson P (2020) Interactions and transport in highly concentrated LiTFSI-based electrolytes. ChemPhysChem 21:1166–1176. https://doi.org/10.1002/cphc.202000153
Devaux D, Bouchet R, Glé D, Denoyel R (2012) Mechanism of ion transport in PEO/LiTFSI complexes: effect of temperature, molecular weight and end groups. Solid State Ionics 227:119–127. https://doi.org/10.1016/j.ssi.2012.09.020
Molinari N, Mailoa JP, Kozinsky B (2018) Effect of salt concentration on ion clustering and transport in polymer solid electrolytes: a molecular dynamics study of PEO–LiTFSI. Chem Mater 30:6298–6306. https://doi.org/10.1021/acs.chemmater.8b01955
Stolwijk NA, Heddier C, Reschke M, Wiencierz M, Bokeloh J, Wilde G (2013) Salt-concentration dependence of the glass transition temperature in PEO–NaI and PEO–LiTFSI polymer electrolytes. Macromolecules 46:8580–8588. https://doi.org/10.1021/ma401686r
Sengwa RJ, Patel VK, Saraswat M (2022) Investigation on promising properties of PEO/PVP/LiTFSI solid polymer electrolytes for high-performance energy storage and next-generation flexible optoelectronic and iontronic devices. J Polym Res 29:480. https://doi.org/10.1007/s10965-022-03326-6
Wetjen M, Kim G-T, Joost M, Appetecchi GB, Winter M, Passerini S (2014) Thermal and electrochemical properties of PEO-LiTFSI-Pyr14TFSI-based composite cathodes, incorporating 4 V-class cathode active materials. J Power Sources 246:846–857. https://doi.org/10.1016/j.jpowsour.2013.08.037
Armand M (1983) Polymer solid electrolytes- an overview. Solid State Ionics. https://doi.org/10.1016/0167-2738(83)90083-8
Newman J, Thomas-Alyea KE (2004) Electrochemical system
Doyle M, Fuller TF, Newman J (1993) Modeling of galvanostatic charge and discharge of the lithium/polymer/insertion cell. J Electrochem Soc 140:1526–1533. https://doi.org/10.1149/1.2221597
Ehrl A, Landesfeind J, Wall WA, Gasteiger HA (2017) Determination of transport parameters in liquid binary lithium ion battery electrolytes. J Electrochem Soc 164:A826–A836. https://doi.org/10.1149/2.1131704jes
Hafezi H, Newman J (2000) Verification and analysis of transference number measurements by the galvanostatic polarization method. J Electrochem Soc 147:3036. https://doi.org/10.1149/1.1393644
Landesfeind J, Ehrl A, Graf M, Wall WA, Gasteiger HA (2016) Direct electrochemical determination of thermodynamic factors in aprotic binary electrolytes. J Electrochem Soc 163:A1254–A1264. https://doi.org/10.1149/2.0651607jes
Ma Y, Doyle M, Fuller TF, Doeff MM, Jonghe LCD, Newman J (1995) The measurement of a complete set of transport properties for a concentrated solid polymer electrolyte solution. J Electrochem Soc 142:1859–1868. https://doi.org/10.1149/1.2044206
Markevich E, Levi MD, Aurbach D (2005) Comparison between potentiostatic and galvanostatic intermittent titration techniques for determination of chemical diffusion coefficients in ion-insertion electrodes. J Electroanal Chem 580:231–237. https://doi.org/10.1016/j.jelechem.2005.03.030
Monroe C, Newman J (2003) Dendrite growth in lithium/polymer systems. J Electrochem Soc 150:A1377. https://doi.org/10.1149/1.1606686
Zhu Y, Wang C (2010) Galvanostatic intermittent titration technique for phase-transformation electrodes. J Phys Chem C 114:2830–2841. https://doi.org/10.1021/jp9113333
Newman J, Chapman TW (1973) Restricted diffusion in binary solutions. AIChE J 19:343–348. https://doi.org/10.1002/aic.690190220
Villaluenga I, Pesko DM, Timachova K, Feng Z, Newman J, Srinivasan V, Balsara NP (2018) Negative stefan-maxwell diffusion coefficients and complete electrochemical transport characterization of homopolymer and block copolymer electrolytes. J Electrochem Soc 165:A2766–A2773. https://doi.org/10.1149/2.0641811jes
Thompson SD, Newman J (1989) Differential diffusion coefficients of sodium polysulfide melts. J Electrochem Soc 136:3362–3369. https://doi.org/10.1149/1.2096451
Wang AA, Hou T, Karanjavala M, Monroe CW (2020) Shifting-reference concentration cells to refine composition-dependent transport characterization of binary lithium-ion electrolytes. Electrochim Acta. https://doi.org/10.1016/j.electacta.2020.136688
Bruce PG, Hardgrave MT, Vincent CA (1992) The determination of transference numbers in solid polymer electrolytes using the Hittorf method. Solid State Ionics 53–56:1087–1094. https://doi.org/10.1016/0167-2738(92)90295-Z
MacInnes DA, Cowperthwaite IA, Blanchard KC (1926) The moving-boundary method for determining transference numbers. V. A constant current apparatus. J Am Chem Soc 2246:1924–1927
Lewis GN (1910) The therory of the determination of transference numbers by the method of moving boundaries. J Am Chem Soc 86:862–869
Longsworth LG, MacInnes DA (1932) Transference numbers by the method of moving boundaries. Chem Rev 4:171–230
Rabette C, Tekaya I, Farkhondeh M, Fleutot B, Delacourt C (2021) Determination of electrolyte transport properties with a multi-reference-electrode cell. J Electrochem Soc 168:060509. https://doi.org/10.1149/1945-7111/ac03f1
Farkhondeh M, Pritzker M, Delacourt C, Liu SSW, Fowler M (2017) Method of the four-electrode electrochemical cell for the characterization of concentrated binary electrolytes: theory and application. J Phys Chem C 121:4112–4129. https://doi.org/10.1021/acs.jpcc.6b11501
Hiller MM, Joost M, Gores HJ, Passerini S, Wiemhöfer HD (2013) The influence of interface polarization on the determination of lithium transference numbers of salt in polyethylene oxide electrolytes. Electrochim Acta 114:21–29. https://doi.org/10.1016/j.electacta.2013.09.138
Utpalla P, Sharma S, Deshpande S, Bahadur J, Sen D, Sahu M, Pujari P (2021) Role of free volumes and segmental dynamics on ion conductivity of PEO/LiTFSI solid polymer electrolytes filled with SiO 2 nanoparticles: a positron annihilation and broadband dielectric spectroscopy study. Phys Chem Chem Phys 23:8585–8597. https://doi.org/10.1039/D1CP00194A
Utpalla P, Sharma S, Sudarshan K, Sahu M, Pujari P (2019) Investigation of the free volume characteristics of PEO based solid state polymer electrolyte by means of positron annihilation spectroscopy. Solid State Ionics. https://doi.org/10.1016/j.ssi.2019.05.025
Marzantowicz M, Dygas JR, Krok F, Łasińska A, Florjańczyk Z, Zygadło-Monikowska E, Affek A (2005) Crystallization and melting of PEO:LiTFSI polymer electrolytes investigated simultaneously by impedance spectroscopy and polarizing microscopy. Electrochim Acta 50:3969–3977. https://doi.org/10.1016/j.electacta.2005.02.053
Meng N, Zhu X, Lian F (2021) Particles in composite polymer electrolyte for solid-state lithium batteries: a review. Particuology 60:14–36. https://doi.org/10.1016/j.partic.2021.04.002
Monroe CW, Delacourt C (2013) Continuum transport laws for locally non-neutral concentrated electrolytes. Electrochim Acta 114:649–657. https://doi.org/10.1016/j.electacta.2013.10.006
Hoffman ZJ, Shah DB, Balsara NP (2021) Temperature and concentration dependence of the ionic transport properties of poly(ethylene oxide) electrolytes. Solid State Ionics 370:115751. https://doi.org/10.1016/j.ssi.2021.115751
Doeff M, Edman L, Sloop S, Kerr J, De Jonghe LC (2000) Transport properties of binary salt polymer electrolytes. J Power Sour. https://doi.org/10.1016/S0378-7753(00)00433-X
Marzantowicz M, Dygas JR, Jenninger W, Alig I (2005) Equivalent circuit analysis of impedance spectra of semicrystalline polymer. Solid State Ionics. https://doi.org/10.1016/j.ssi.2004.12.018
Tien. Quang. Nguyen, Cornelia. Breitkopf, (2018) Determination of diffusion coefficients using impedance spectroscopy data. J Electrochem Soc 165:E826–E831. https://doi.org/10.1149/2.1151814jes
Andrzej L (2014) Electrochemical impedance spectroscopy and its applications. Springer, Berlin
Meddings N, Heinrich M, Overney F, Lee JS, Ruiz V, Napolitano E, Seitz S, Hinds G, Raccichini R, Gaberšček M, Park J (2020) Application of electrochemical impedance spectroscopy to commercial Li-ion cells: a review. J Power Sour. https://doi.org/10.1016/j.jpowsour.2020.228742
Pesko DM, Timachova K, Bhattacharya R, Smith M, Villaluenga I, Newman J, Balsara NP (2017) Negative transference numbers in poly(ethylene oxide)-based electrolytes. J Electrochem Soc 164:E3569–E3575. https://doi.org/10.1149/2.0581711jes
Gao KW, Balsara NP (2021) Electrochemical properties of poly(ethylene oxide) electrolytes above the entanglement threshold. Solid State Ionics. https://doi.org/10.1016/j.ssi.2021.115609
Galluzzo MD, Loo WS, Wang AA, Walton A, Maslyn JA, Balsara NP (2020) Measurement of three transport coefficients and the thermodynamic factor in block copolymer electrolytes with different morphologies. J Phys Chem B 124:921–935. https://doi.org/10.1021/acs.jpcb.9b11066
Galluzzo MD, Halat DM, Loo WS, Mullin SA, Reimer JA, Balsara NP (2019) Dissolution of lithium metal in poly(ethylene oxide). ACS Energy Lett 4:903–907. https://doi.org/10.1021/acsenergylett.9b00459
Hashim NHAM, Subban RHY (2018) Studies on conductivity, structural and thermal properties of PEO-LiTFSI polymer electrolytes doped with EMImTFSI ionic liquid. AIP Conf Proc. https://doi.org/10.1063/1.5066977
Ratner MA, Nitzan A (1989) Conductivity in polymer ionics. Faraday Discuss Chem Soc 88:19–42
Armand M (1994) The history of polymer electrolytes. Solid State Ionics 69:309–319. https://doi.org/10.1016/0167-2738(94)90419-7
Lascaud S, Perrier M, Vallée A, Besner S, Prudʼhomme J, Armand M (1994) Phase diagrams and conductivity behavior of poly(ethylene oxide)-molten salt rubbery electrolytes. Macromolecules 27:7469–7477. https://doi.org/10.1021/ma00103a034
Mongcopa KIS, Tyagi M, Mailoa JP, Samsonidze G, Kozinsky B, Mullin SA, Gribble DA, Watanabe H, Balsara NP (2018) Relationship between segmental dynamics measured by quasi-elastic neutron scattering and conductivity in polymer electrolytes. ACS Macro Lett 7:504–508. https://doi.org/10.1021/acsmacrolett.8b00159
Armand MB, Bruce PG, Forsyth M, Scrosati B, Wieczorek W (2011) Polymer electrolytes. In: Bruce DW, O’Hare D, Walton RI (eds) Energy materials, 1st edn. Wiley, New York, pp 1–31
Brandell D, Priimägi P, Kasemägi H, Aabloo A (2011) Branched polyethylene/poly(ethylene oxide) as a host matrix for Li-ion battery electrolytes: a molecular dynamics study. Electrochim Acta 57:228–236. https://doi.org/10.1016/j.electacta.2011.03.022
Monroe CW, Newman J (2006) Onsager reciprocal relations for stefan-maxwell diffusion. Ind Eng Chem Res 45:5361–5367. https://doi.org/10.1021/ie051061e
Aniya M, Kawamura J (2002) Medium range structure and activation energy of ion transport in glasses. Solid State Ionics 154–155:343–348. https://doi.org/10.1016/S0167-2738(02)00571-4
Aniya M (2008) Medium range structure and power law conductivity dispersion in superionic glasses. J Non-Cryst Solids 354:365–369. https://doi.org/10.1016/j.jnoncrysol.2007.06.088
Acknowledgements
This work was financially supported by to ANRT « Association Nationale de la Recherche et de la Technologie » and RENAULT SA (Renault Technocentre, 78084 Guyancourt, France).
Author information
Authors and Affiliations
Contributions
ST: Conceptualization, Software, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Writing - original draft preparation. FC: Conceptualization, Formal analysis, Investigation, Validation, Writing - review & editing. LL: Conceptualization, Formal analysis, Investigation, Validation, Writing- review & editing. J-CR: Conceptualization, Formal analysis, Investigation, Validation, Methodology, Writing - review & editing. TT: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Corresponding authors
Ethics declarations
Competing interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Toe, S., Chauvet, F., Leveau, L. et al. Impact of unsolvated lithium salt concentration on the ions transport pathway in polymer electrolyte (LiTFSI-PEO): empirical mathematical model to predict the ionic conductivity. J Appl Electrochem 53, 1939–1951 (2023). https://doi.org/10.1007/s10800-023-01900-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-023-01900-4