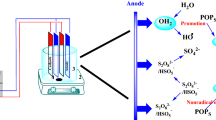
Abstract
In this study, the efficiency of different electrode pairs in the treatment of strained yoghurt wastewater with electrochemical oxidation method and the operating conditions in the reactor (distance between electrodes and applied potential) in specific energy consumption (SEC) for COD removal were investigated by applying the Taguchi experimental design and ANOVA analysis. For different electrode pairs, the highest COD removal was obtained as 24.79–28.9%, and it was observed that the electrode type did not have a significant effect on organic matter removal. It has been found that the operating conditions for different electrodes show similar trends for SEC in COD removal. The applied potential for all electrodes was determined as the most effective operating condition, followed by the reaction time and the distance between the electrodes. According to the results of ANOVA, the correlation coefficients of different electrode pairs were determined in the range of 82.33–91.21% for the SEC in COD removal. SEC under the studied experimental conditions is between the following values: 0.0019–2.5695, 0.0011–0.5702, 0.0007–0.5721, and 0.012–0.3641 kW h kg COD−1 for steel (anode)–steel (cathode), graphite (anode)–graphite (cathode), graphite (anode)–steel (cathode), and BDD (anode)–graphite (cathode) electrode pairs, respectively. It is thought that the electrochemical oxidation process will be a good alternative treatment method for strained yoghurt wastewater, which has a very high COD content and low pH value, as a post-treatment unit or in combination with other methods, due to an effective COD removal at low reaction times and low SEC.
Graphical Abstract




Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article.
Abbreviations
- E:
-
Specific energy consumption
- EO:
-
Electrochemical oxidation
- EOP:
-
Electrochemical oxidation process
- COD:
-
Chemical oxygen demand
- YW:
-
Yoghurt whey
- SYW:
-
Strained yoghurt wastewater
- BDD:
-
Boron doped diamond
References
Un UT, Ozel E (2013) Electrocoagulation of yogurt industry wastewater and the production of ceramic pigments from the sludge. Sep Purif Technol 120:386–391
Öztürk Ö (2020) Investigating the suitability of strained yoghurt acid whey for pickling. Dissertation, Sakarya University
Kucukcongar S, Gok Z, Oden M, Dursun S (2022) Biodegradability of dissolved organic nitrogen in yoghurt and cheese production wastewaters. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-022-04434-y
Carvalho F, Prazeres AR, Rivas J (2013) Cheese whey wastewater: characterization and treatment. Sci Total Environ 445:385–396
Mortezaei Y, Amani T, Elyasi S (2018) High-rate anaerobic digestion of yogurt wastewater in a hybrid EGSB and fixed-bed reactor: optimizing through response surface methodology. Process Saf Environ Prot 113:255–263
Luo H, Xu G, Lu Y, Liu G, Zhang R, Li X et al (2017) Electricity generation in a microbial fuel cell using yogurt wastewater under alkaline conditions. RSC Adv 7(52):32826–32832
Kadji H, Yahiaoui I, Akkouche F, Boudrahem F, Ramdani S, Saidane A et al (2022) Heterogeneous degradation of amoxicillin in the presence of synthesized alginate-Fe beads catalyst by the electro-Fenton process using a graphite cathode recovered from used batteries. Water Sci Technol 85(6):1840–1854
Kadji H, Yahiaoui I, Garti Z, Amrane A, Aissani-Benissad F (2021) Kinetic degradation of amoxicillin by using the electro-Fenton process in the presence of a graphite rods from used batteries. Chin J Chem Eng 32:183–190
Mohan N, Balasubramanian N, Basha CA (2007) Electrochemical oxidation of textile wastewater and its reuse. J Hazard Mater 147(1–2):644–651
Liu X, Chen Z, Du W, Liu P, Zhang L, Shi F (2022) Treatment of wastewater containing methyl orange dye by fluidized three dimensional electrochemical oxidation process integrated with chemical oxidation and adsorption. J Environ Manage 311:114775
Li Y, Lu D, Liu X, Li Z, Zhu H, Cui J et al (2022) Coupling of cathodic aluminum dissolution and anodic oxidation process for simultaneous removal of phosphate and ammonia in wastewaters. Chem Eng J 427:130944
Lu S, Zhang G (2022) Recent advances on inactivation of waterborne pathogenic microorganisms by (photo) electrochemical oxidation processes: Design and application strategies. Journal of Hazardous Materials 431:128619
Garcia-Segura S, Ocon JD, Chong MN (2018) Electrochemical oxidation remediation of real wastewater effluents—a review. Process Saf Environ Prot 113:48–67
Rao NN, Somasekhar KM, Kaul SN, Szpyrkowicz L (2001) Electrochemical oxidation of tannery wastewater. J Chem Technol Biotechnol 76(11):1124–1131
Gargouri B, Gargouri OD, Khmakhem I, Ammar S, Abdelhèdi R, Bouaziz M (2017) Chemical composition and direct electrochemical oxidation of table olive processing wastewater using high oxidation power anodes. Chemosphere 166:363–371
Klidi N, Clematis D, Delucchi M, Gadri A, Ammar S, Panizza M (2018) Applicability of electrochemical methods to paper mill wastewater for reuse. Anodic oxidation with BDD and TiRuSnO2 anodes. J Electroanal Chem 815:16–23
Zheng T, Wang Q, Shi Z, Fang Y, Shi S, Wang J et al (2016) Advanced treatment of wet-spun acrylic fiber manufacturing wastewater using three-dimensional electrochemical oxidation. J Environ Sci 50:21–31
Sastrawidana IDK, Sukarta IN (2018) Indirect electrochemical oxidation with multi carbon electrodes for restaurant wastewater treatment. J Ecol Eng. https://doi.org/10.12911/22998993/79414
Tirado L, Gökkuş Ö, Brillas E, Sirés I (2018) Treatment of cheese whey wastewater by combined electrochemical processes. J Appl Electrochem 48(12):1307–1319
Jawad SS, Abbar AH (2019) Treatment of petroleum refinery wastewater by electrochemical oxidation using graphite anodes. Al-Qadisiyah J Eng Sci 12(3):144–150
Valero D, García-García V, Expósito E, Aldaz A, Montiel V (2014) Electrochemical treatment of wastewater from almond industry using DSA-type anodes: direct connection to a PV generator. Sep Purif Technol 123:15–22
Li W, Zhou Q, Hua T (2010) Removal of organic matter from landfill leachate by advanced oxidation processes: a review. Int J Chem Eng. https://doi.org/10.1155/2010/270532
Taguchi G, Wu Y (1989) Taguchi methods: case studies from the US and Europe. American Supplier Institute Inc., Michigan
Taguchi G (1986) Introduction to quality engineering: designing quality into products and processes. White Plains: Asian Productivity Organization/UNIPUB, Tokyo, Japan
Hiwarkar AD, Chauhan R, Patidar R, Srivastava VC, Singh S, Mall ID (2021) Binary electrochemical mineralization of heterocyclic nitrogenous compounds: parametric optimization using Taguchi method and mineralization mechanism. Environ Sci Pollut Res 28(6):7332–7346
Ibrahim HM, Salman RH (2022) Real wastewater treatment by electrocoagulation-electro-oxidation combined system: optimization using Taguchi approach. Egypt J Chem 65(3):1–2
Keshavarz Moraveji M, Malekinejad N, Joudaki E (2012) Oil removal from an oil-in-water emulsion by electrochemical process using Taguchi method. Desalin Water Treat 49(1–3):19–25
He Y, Lin H, Guo Z, Zhang W, Li H, Huang W (2019) Recent developments and advances in boron-doped diamond electrodes for electrochemical oxidation of organic pollutants. Sep Purif Technol 212:802–821
Ozturk D, Yilmaz A, Sapci AZ (2021) Electrochemical mineralization of abattoir wastewater with continuous system. Int J Environ Sci Technol 18(12):3761–3776
Murthy UN, Rekha H, Bhavya J (2011) Performance of electrochemical oxidation in treating textile dye wastewater by stainless steel anode. Int J Environ Sci Develop 2(6):483
Struk-Sokołowska J, Rodziewicz J, Mielcarek A (2017) (2018) Effect of dairy wastewater on changes in COD fractions in technical-scale SBR type reactors. Water Sci Technol 1:156–169
Seçkin A. (1996) Süzme yoğurt üretimi sırasında yoğurttaki besin öğelerinde meydana gelen kayıplar üzerine araştırmalar. Celal Bayar Ü Fen Bil Enst. Fen Bilimleri Enstitüsü, Yüksek Lisans Tezi, Manisa.
Kose S, Kose YE, Altun I (2019) A study on mineral content of whey obtained from Turkish strained yogurt. Comptes Rendus de I Academie Bulgare des Sci 72:1732–1738
Kirdar SS, Toprak G, Guzel A (2017) Determination of the mineral content in yogurt whey. Eur Int J Sci Technol 6:26–34
Keser AR, Kirdar SS (2020) Determination of the pollution parameters in strained yogurt industry wastewater. Eur J Adv Eng Technol 7(9):5–11
Güven G, Perendeci A, Tanyolaç A (2008) Electrochemical treatment of deproteinated whey wastewater and optimization of treatment conditions with response surface methodology. J Hazard Mater 157(1):69–78
Chen G (2004) Electrochemical technologies in wastewater treatment. Sep Purif Technol 38(1):11–41
Kashi G (2017) Optimization of electrochemical process for phenanthrene removal from aqueous medium by Taguchi. Toxicol Environ Chem 99(5–6):772–782
Bhatnagar R, Joshi H, Mall ID, Srivastava VC (2014) Electrochemical oxidation of textile industry wastewater by graphite electrodes. J Environ Sci Health Part A 49(8):955–966
Ozturk D, Dagdas E, Fil BA, Bashir MJ (2021) Central composite modeling for electrochemical degradation of paint manufacturing plant wastewater: one-step/two-response optimization. Environ Technol Innov 21:101264
Saleh M, Yildirim R, Isik Z, Karagunduz A, Keskinler B, Dizge N (2021) Optimization of the electrochemical oxidation of textile wastewater by graphite electrodes by response surface methodology and artificial neural network. Water Sci Technol 84(5):1245–1256
Ozturk D, Yilmaz AE (2019) Treatment of slaughterhouse wastewater with the electrochemical oxidation process: role of operating parameters on treatment efficiency and energy consumption. J Water Process Eng 31:100834
Körbahti BK, Aktaş N, Tanyolaç A (2007) Optimization of electrochemical treatment of industrial paint wastewater with response surface methodology. J Hazard Mater 148(1–2):83–90
Bensadok K, El Hanafi N, Lapicque F (2011) Electrochemical treatment of dairy effluent using combined Al and Ti/Pt electrodes system. Desalination 280(1–3):244–251
Sivagami K, Vikraman B, Krishna RR, Swaminathan T (2016) Chlorpyrifos and endosulfan degradation studies in an annular slurry photo reactor. Ecotoxicol Environ Saf 134:327–331
Singh S, Singh S, Lo SL, Kumar N (2016) Electrochemical treatment of Ayurveda pharmaceuticals wastewater: optimization and characterization of sludge residue. J Taiwan Inst Chem Eng 67:385–396
Johnson I, Kumar M (2020) Electrochemical oxidation of distillery wastewater by dimensionally stable Ti-RuO2 anodes. Environ Technol Innov 20:101181
Özçelep B. (2009) Kağıt endüstrisi atıksularının membran prosesleriyle ileri arıtımı. Doktora Tezi, İstanbul Teknik Üniversitesi, İstanbul.
Land CE (1971) Confidence intervals for linear functions of the normal mean and variance. Ann Math Stat 42(4):1187–1205
Darvishmotevalli M, Zarei A, Moradnia M, Noorisepehr M, Mohammadi H (2019) Optimization of saline wastewater treatment using electrochemical oxidation process: prediction by RSM method. MethodsX 6:1101–1113
Funding
This study is financially supported by Selçuk University Scientific Research Projects Coordinating Office under Grant No 20401133.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by ZG, SK, MT, and STO. The first draft of the manuscript was written by ZG, SK, MT, and STO, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gok, Z., Kucukcongar, S., Turkyilmaz, M. et al. Treatment of strained yoghurt wastewater by electrochemical oxidation method using Taguchi experimental design. J Appl Electrochem 53, 1595–1607 (2023). https://doi.org/10.1007/s10800-023-01866-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-023-01866-3