Abstract
The inhibitive action of 2-aminobenzothiazole (ABT) on the corrosion of AA6061-T6 was evaluated in 0.5 M HCl by electrochemical techniques. The electrochemical results were validated by theoretical calculations using density functional theory (DFT). ABT showed a mixed inhibitor behaviour with 71–90% inhibition efficiency in the 1 × 10−4 to 1 × 10−3 M concentration range and at 303 to 323 K temperature. The inhibition power of ABT increases with its concentration and rise in the temperature of the medium. The polarization results showed a reduction in corrosion rate and improvement in inhibition performance on increasing ABT concentrations, which reveal the ABT's adsorption on the alloy. The evaluation of kinetic and thermodynamic results revealed that ABT inhibits the AA6061-T6 corrosion by mixed adsorption, following the Langmuir isotherm model. The observed increase in polarization resistance with increased ABT concentrations indicates the attenuation of AA6061-T6 deterioration. Furthermore, the corroded and inhibited specimen's surface scanning is performed to confirm the ABT's adsorption on the alloy sample by scanning electron microscopy (SEM) and atomic force microscopy (AFM) techniques.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
AA6061 is an alloy of Al with Mg and Si as the primary alloying materials. It is commercially available in different grades: pre-tempered/annealed (AA6061-O), tempered-solutionized and artificially aged (AA6061-T6), and solutionized, stress-relieved, stretched and artificially aged (AA6061-T651) [1]. Aluminium alloys show high technological and industrial values. Hence, they are widely used in automobiles, aircraft, chemical reactors, and batteries [2]. Al and its alloys usually develop a passive oxide film at the surface on exposure to air. Such a passive material on exposure to an aggressive environment, such as acids, chlorides, and alkalis, could break the protective oxide film and eventually cause corrosion of the material [3, 4]. Among different acids, hydrochloric acid solutions are widely employed as chemical etchants in the Al alloys industry. In such critical conditions, the usage of inhibitors is the simplest and most cost-effective method to control corrosion of these materials [5]. As reported, organic compounds with heteroatoms and multiple double bonds are the most effective inhibitors [6, 7]. 2-Aminobenzothiazole (ABT) is one such compound, which has not been explored fully as an effective inhibitor for different metals/alloys. The survey of literature has revealed the excellent corrosion inhibition activity of ABT on steel in 1 M H2SO4 [8], carbon steel [9], and mild steel in 1 M HCl [10]. ABT is readily available and soluble in an aqueous acid medium (0.5 M HCl). Hence, there is no need to use an additional organic solvent to prepare its solution, avoiding possible environmental pollution. This paper reports the inhibition behaviour of ABT in 0.5 M HCl medium on AA6061-T6 corrosion. The results of electrochemical studies were validated by surface morphological studies and theoretical studies by DFT calculations.
2 Experimental procedure
2.1 Materials
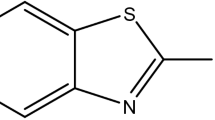
A cylindrical coupon of AA6061-T6 with elemental compositions (wt%): Si (0.51), Mg (0.82), Mn (0.15), Cu (0.1), Zn (0.25), Cr (0.04), and Al (98.05) embedded in epoxy resin with an exposed area of 1 cm2. The sample's exposed surface is abraded with different emery papers (like 220, 400, 600, and 1000) and a disc polishing wheel. Finally, the alloy specimen was cleaned in water, acetone rinsed, and dried. The corrosive medium of 0.5 M hydrochloric acid prepared from the AnalaR grade sample (37%) was finally standardized using a volumetric method. ABT (Merck) was employed as the inhibitor material. Its solution of required concentration in distilled water was prepared and used. Figure 1 shows the chemical structure of ABT.
2.2 Electrochemical studies
Electrochemical measurements were performed using a CH Instrument assisted with beta software (CH600E-series, U.S. model). The experiments were conducted using a three-electrode assembly of saturated calomel (SCE), platinum, and an Al-6061-T6 specimen dipped in 0.5 M HCl medium at 303, 313, and 323 K. The connected setup was allowed to stand for about an hour to reach the steady-state OCP (open circuit potential). EIS (electrochemical impedance study) measurements were taken under an applied AC signal of 10 mV amplitude by varying the frequency from 10 kHz to 0.01 Hz at OCP. In PDP (potentiodynamic polarization) measurements, the working electrode of the AA6061-T6 specimen was applied with varying potential from − 250 mV cathodic to + 250 mV anodic at a 1 mV s−1 scan rate.
2.3 Surface morphological studies
The surface morphology of the specimen is essential in analyzing the surface changes that occur before and after inhibition. The surface images of alloy specimens were recorded using scanning electron microscopy (SEM, EVO 18-5-57 model). The alloy specimen was immersed in a 0.5 M HCl for about 3 h, and its SEM image was taken. A similar procedure was repeated by immersing the sample in 0.5 M HCl containing 1 × 10−3 M ABT for 3 h; the SEM image was recorded. The surface roughness of corroded and inhibited alloy specimen was checked using an IB342-Innova model AFM.
2.4 Theoretical calculations
The inhibitor (ABT) molecule was subjected to DFT (Density functional theory) treatment using Schrodinger's material science suit with B3LYP as exchange–correlation function and 6-31G** as the basis set [11]. The different theoretical parameters like EHOMO and ELUMO (energies of highest occupied and lowest unoccupied molecular orbitals), ∆Eg (energy gap), χ (electronegativity), η (hardness), σ (softness), ε (electrophilicity), and ΔN (the fraction of electron transferred) were computed to validate the inhibition performance of the inhibitor.
3 Results and discussion
3.1 PDP results
The variation of potential with time for AA6061-T6 in 0.5 M HCl without and with 5.0 × 10−4 M ABT at 313 K is shown in Fig. 2. From Fig. 2, it is seen that the potential of alloy in 0.5 M HCl moves towards more negative side up to 2000s and remains stable at around − 0.715 V (SCE) with the increase in immersion time. In the presence of 5.0 × 10−4 M ABT, the potential value moves towards a more negative side up to the 2000s and remains stable at around − 0 711 V on increasing immersion time. This trend indicates that ABT acts as a mixed-type inhibitor.
The PDP plots recorded for AA6061-T6 corrosion in a 0.5 M HCl containing different ABT concentrations at 313 K are presented in Fig. 3. Similar PDP plots were obtained at different studied temperatures (303 and 323 K). The anodic curve pertains to metal oxidation, whereas the cathodic curve refers to the hydrogen release reaction occurring in the acid medium [12]. The parameters such as Ecorr (corrosion potential, V), icorr (corrosion current density, A cm−2), and βc and βa (cathodic and anodic Tafel slopes, V dec−1) were obtained from this plot.
The corrosion rate (CR) of the specimen alloy in mmpy, surface coverage (ϴ), and inhibition efficiency of ABT in percentage (% IE) were computed as per the relations [Eqs. (1)–(3)], respectively [13]
3270 is equal to the unit conversion factor, ρ denotes the density of specimen metal, Z indicates the number of electrons transferred, and M represent the metal's atomic mass
icorr(inh) and icorr indicate the observed value of the corrosion current densities in the inhibited and blank medium
Due to increased medium conductance, both icorr and CR values increased with temperature in the blank medium. It is clear from Table 1 that there is no significant change in Ecorr values in the inhibited medium than in the blank medium. However, as the concentration of ABT increases, the anodic and cathodic curves of the PDP plots (Fig. 3) shift to lower icorr values, and Ecorr values move towards the anodic or cathodic directions compared to the blank acid, revealing the mixed-type behaviour of ABT. As per the literature [14], if the observed change in Ecorr value in the acid medium-containing inhibitor exceeds ± 85 mV than in the acid medium, then the employed inhibitor can act as a cathodic or anodic type. In the present work, the change in Ecorr value is within ± 85 mV; hence, ABT could be a mixed-type inhibitor. With the increase in ABT concentration, there is a drastic decrease in icorr value leading to effective CR control and increasing the inhibitor's inhibition efficiency at all the studied temperatures. It is apparently due to the protective film formation by adsorption of ABT molecules, which protects the alloy from corrosion [14]. In the PDP method, ABT evinced a maximum inhibition efficiency of 86.62% at 323 K for 1.0 × 10−3 M concentration. In addition, the value of βc does not vary much on increasing the ABT concentration (Table 1). It shows that adding ABT raises the energy barrier for the proton discharge reaction, controlling the cathodic reaction. Similarly, no significant shift in the βa value is observed on increasing ABT concentration. Thus, the added ABT controls the deterioration of the alloy sample by blocking the cathodic regions without altering the inhibition mechanism [15, 16].
3.2 EIS results
EIS is one of the main techniques employed to study the electrochemical process characteristics at the metal/solution interface. Nyquist plots obtained for AA6061-T6 in a 0.5 M HCl containing varying ABT concentrations at 313 K are shown in Fig. 4a. The diameter of semicircles increases with an increase in ABT concentration, revealing a reduction in CR due to the adsorption of ABT molecules on the surface of the alloy. An identical Nyquist plot type was observed at other studied temperatures (303 and 323 K). A similar pattern of Nyquist plots was reported earlier for Al alloys and their composites in the HCl medium [17,18,19]. The Nyquist plot depicts a capacitive loop at a higher frequency (HF), while an inductive loop at a lower frequency (LF) region. The HF loop indicates the resistance offered to the charge transfer during the corrosion process due to the formation of protective oxide film over the alloy surface. On the other hand, the LF loop corresponds to the relaxation process of H+ ions’ and Cl− ions’ adsorption onto the oxide film [20]. Aluminium is first oxidised to Al+ ion, which migrates towards the oxide/acid medium interface, forming Al3+ ion and releasing O2− or OH− ions. A single capacitive loop may represent these processes, because they overlap or one process dominates the others [21].
Based on the shapes of the Nyquist plots, the impedance data analysis was done by choosing a suitable equivalent circuit. The fitment of the impedance results obtained an appropriate equivalent circuit containing five elements using ZSimpwin software (3.21 version). Figure 4b depicts the simulated plot and equivalent circuit. From Table 2, it is clear that the χ2 values obtained are in the order of 10−3, which supports the validity of the used equivalent circuit. The equivalent circuit consists of a solution resistance component (RS) and a constant phase element (Q). The resistor RS is in series to Rct (charge transfer resistance) and Q, while Rct is parallel to Q. RL and L are the inductive elements, which are in series.
Nyquist plots (Fig. 4a) obtained are depressed semicircles, commonly known as frequency dispersion resulting from the inhomogeneous surface nature, such as surface roughness, dislocations, etc. [22]. Mansfeld et al. [23] have proposed an exponent, n, in the impedance function as a deviation parameter from the ideal behaviour. Based on this, the capacitor in the equivalent circuit could be replaced by a surface-related frequency-dependent element (CPE). The impedance function of CPE can be represented by Eq. (4) [24]
where A is the constant related to the metal surface and electropositive species, ω is the angular frequency, i is an imaginary number, and n is an exponent value (− 1 ≤ n ≤ 1), which depends on the surface morphology and reflects the microscopic variations of the metal surface. The CPE acts like an ideal capacitor when n = 1. In the present work (Table 2), the observed n values for ABT were between 0.83 and 0.96. The higher n value means lower surface roughness, presumably indicating the formation of a protective film by ABT on the alloy surface [25].
The double-layer capacitance (Cdl) values for a circuit, including CPE, were computed using Eq. (5)
fmax indicates the frequency at which the imaginary component of the Nyquist plot is maximum [26].
Polarization resistance (Rp) value is calculated as
The % IE is computed as per the equation
Rp and Rp(inh) represent the polarization resistances in the acid and inhibited acid medium, respectively [17].
From Table 2, it is evident that Rp values increased with the rise in the ABT concentration. It may be because the inhibitor film formation on the alloy surface increases the electron flow resistance. The decrease in Cdl value with a rise in ABT concentration may be due to the increase in electrical double-layer thickness at the alloy/medium junction [27] and the displacement of adsorbed water molecules at the alloy surface by ABT molecules [28]. It suggests that the ABT controls the corrosion of AA6061-T6 alloy due to its adsorption at the alloy/medium interface. ABT showed a maximum % IE of 90.47 at the concentration of 1.0 × 10−3 M and 323 K.
3.3 Influence of temperature
Based on the variation of CR and % IE with temperature, kinetic and thermodynamic results were evaluated for the corrosion/inhibition process. These results become significant while interpreting the ABT's adsorption behaviour. Generally, the conductivity of the acid medium tends to increase with a rising temperature, leading to an increase in CR. The added inhibitor brings down the CR to the minimum level and provides the necessary protection to the alloy. The observed improvement in the inhibition performance of ABT in 0.5 M HCl on increasing the temperature of the medium indicates that inhibition occurs predominantly by chemisorption at higher temperatures [27]. From the Arrhenius equation [Eq. (8)], Ea (activation energy) for AA6061-T6 corrosion in the hydrochloric acid medium is computed
In this relation, R denotes the gas constant, while B indicates the Arrhenius constant [28].
The Arrhenius plot for AA6061-T6 is shown in Fig. 5. This plot's slope equals − Ea/R. Hence, the Ea values were obtained.
The enthalpy (∆H#) and entropy (∆S#) of activation for the corrosion process in the blank and inhibited medium were calculated from the transition state equation [Eq. (9)]
N and h represent Avogadro's number and Planck's constant, respectively [29].
Figure 6 shows a straight, obtained by plotting ln (CR/T) versus 1/T. The intercept of the plot is given by [ln(R/Nh) + ∆S#/R], and its slope by (− ∆H#/R). The obtained activation parameters are recorded in Table 3.
According to Szauer and Brandt [30], higher activation energy in the presence of an inhibitor is an indication of physisorption. In comparison, lower activation energy is interrelated to the existence of chemisorption. In the present case, the observed Ea value in the presence of ABT in the acid medium is lower than in its absence (Table 3), revealing the chemisorption of ABT molecules on the alloy surface. The increase in % IE of ABT with rising temperature also supports its chemisorption [27]. Similar trends in activation energy are also reported earlier [31,32,33]. The higher value of ∆S# in the inhibited medium reveals the increase in disorders on going from reactant to product species because of the entropy increase of the medium during the adsorption process [34].
3.4 Adsorption isotherm
The nature of the interaction of inhibitor molecules on the metal surface could be more apparent by applying the experimental results to different adsorption isotherm models. The influence of inhibitor concentration on its degree of surface coverage (Ɵ) was confirmed by fitting the experimental results with various adsorption isotherms. In this case, the best fitment observed for adsorption with the Langmuir isotherm model.
Langmuir's adsorption isotherm is represented by Eq. (10)
Cinh indicates the inhibitor concentration, and Kads represents the equilibrium constant for adsorption, the reciprocal of the intercept of the straight line in Langmuir's plot (Fig. 7) [35]. The straight-line plots obtained with regression coefficient (R2) values almost equal to one indicate that ABTs’ adsorption follows Langmuir's adsorption isotherm.
The standard free energy for adsorption of ABT on the alloy surface, ΔGoads, is related to Kads as per Eq. (11)
Here, 55.5 mol dm−3 represents the water concentration in the solution [36].
The plot of ∆Goads versus T is a straight line, as represented in Fig. 8. The straight-line plot's slope and regression coefficient (R2) values are closer to one. The straight line's slope and intercept give the standard value of enthalpy (ΔHoads) and entropy (ΔSoads) of adsorption. ∆Goads value is obtained by Eq. (12)
The larger values of Kads (Table 4) show a stable inhibitor film formation on the alloy surface, resulting in a higher inhibition performance [37]. It also indicates the possibility of a strong interaction of ABT, resulting in chemisorption [14]. The ∆Goads values (Table 4) for ABT adsorption on the alloy surface are between − 20 and − 40 kJ mol−1, and the observed values are closer to − 40 kJ mol−1. Thus, ABT follows mixed adsorption, mainly undergoing chemisorption [38]. The positive value of ∆Hoads confirms that adsorption occurs predominantly through chemisorption [27]. The negative value of ∆Soads indicates the reduction in disorderliness occurring during the transfer of the reactant to the adsorbed species [39].
3.5 Mechanism of inhibition
The inhibitive action of ABT in 0.5 M HCl against AA6061-T6 corrosion depends on its adsorption over the alloy surface. As per ∆Goads values, it is clear that ABT molecules predominantly undergo mixed adsorption with chemisorption. Generally, organic compounds as inhibitors control the corrosion of metals/alloys through the adsorption process, which may occur by the physisorption, chemisorption, or both types of adsorption of the inhibitor molecules on the metal/alloy surface. The extent of adsorption depends on the nature of the metal and its surface texture, the inhibitor's chemical structure, and the aggressiveness and temperature of the medium.
The heteroatoms (N and S) and π-bonding electrons of the aromatic ring in ABT certainly favour its chemisorption on the alloy surface (Fig. 9). The ABT's polar functional group (–NH2) and the conjugated π-electrons of the aromatic ring can act as the active adsorption centres, resulting in stronger adsorption [6, 7]. The planarity of ABT molecules may attribute to the higher inhibition efficiency because of its effective adsorption covering a larger surface area of the alloy [40].
The electrochemical corrosion of aluminium in contact with the hydrochloric acid solution can be represented by the following reactions [41]:
Anodic reaction:
Cathodic reaction:
Generally, the surface of a metal in contact with an electrolyte solution acquires an electric charge due to the development of an electric field at the metal/medium boundary. At the zero charge potential, the pHZch value for aluminium metal is 9.1 [42]. Hence, in a highly acidic medium similar to the one used in this work, aluminium acquires a positive charge. As a result, the negatively charged chloride ions of the acid medium are adsorbed first on the positively charged alloy surface through the electrostatic forces of attraction. In an acidic medium, the amino group of ABT may undergo protonation to form ABTH+. The chloride ions pre-adsorbed on the alloy surface can electrostatically attract ABTH+, resulting in physisorption.
3.6 SEM analysis
Figure 10a, b presents SEM images of corroded and inhibited alloy surfaces. The corroded surface of the alloy in 0.5 M HCl shows many micro-pits and exposes a rough surface. However, the inhibited alloy surface shows fewer micro-holes and exposes a levelled surface. The differences in corroded and inhibited alloy's surface texture indicate the adsorbed layer formation by ABT on the alloy. Thus, the added inhibitor, ABT, protects the alloy by forming an adsorbed layer.
3.7 AFM analysis
Atomic force microscopy (AFM) analysis of the corroded and inhibited alloy surface can provide the extent of roughness on the specimen. Figure 11a, b indicates corroded and inhibited alloy surface AFM images. The AFM pictures show a smoother surface for the inhibited specimen (Fig. 11b) compared to the rougher texture of the corroded sample (Fig. 11a). The observed surface roughness, the average (Ra), and root-mean-square (Rq) value of the inhibited specimen are much less than the corroded sample (Table 5). It confirms the ABT molecule's adsorption on the AA6061-T6 sample, forming a protective film.
3.8 Quantum chemical theoretical study
Generally, the inhibition of corrosion occurs by the inhibitor molecule's adsorption on the metal surface. Therefore, the inhibition performance of an organic inhibitor depends mainly on the extent of its adsorption, which is related to its physicochemical properties. These properties include the chemical structure of the inhibitor with active functional groups, heteroatoms, π bonds, and its mode of adsorption. The influence of physicochemical properties on the adsorption of an organic inhibitor could be analyzed by quantum chemical calculations [43]. These parameters play essential roles in the analysis of inhibition activity. The Mulliken's charge on the atoms in the inhibitor molecule indicates the active adsorption centres of interaction with the metal surface.
The inhibitor (ABT) used in this work can exist in two tautomeric forms (Fig. 12): amino (2-aminobenzothiazole) and imino (2-iminobenzothiazole) [44]. Furthermore, the inhibitor can undergo protonation at the nitrogen atom of the amino group in an acidic medium similar to the one used in this work [45]. Therefore, ABT's amino, imino, and protonated forms are subjected to theoretical studies based on DFT using the Schrodinger quantum chemical suit with a 6-31G** basis set.
In an inhibitor molecule, heteroatoms with high negative Mulliken charges can act as active adsorption sites. From Fig. 13, it is evident that atoms such as N10 (− 0.64) and N7 (− 0.50) in amino; N10 (− 0.60) and N7 (− 0.56) in imino; N10 (− 0.57) and N7 (− 0.41) in protonated form of the inhibitor are the preferred active adsorption sites.
The optimized molecular structures of the inhibitor's amino, imino, and protonated form and their respective HOMO and LUMO are presented in Fig. 14a–i.
The parameters, such as EHOMO, ELOMO, ∆Eg, χ, η, and σ, were computed [45, 46] and are tabulated in Table 6.
EHOMO value measures the electron-releasing tendency, while ELUMO values an inhibitor molecule's electron-accepting ability. The inhibitor molecule's interaction at the alloy surface improves with higher EHOMO and lower ELUMO values [47]. Table 6 shows that the imino form of the inhibitor showed the highest EHOMO value (− 5.526 eV), and the protonated inhibitor exhibited the lowest ELUMO value (− 5.148 eV). Therefore, the imino inhibitor is expected to show a higher tendency to release electrons to the d-orbitals of the Al atom, forming a coordinate type bond. The protonated inhibitor can readily accept electrons from the antibonding orbitals of the Al atom, resulting in back bonding. It leads to better adsorption of inhibitor molecules causing higher inhibition performance [48]. It indicates that inhibition occurs through mixed adsorption of inhibitor molecules, as revealed earlier by the thermodynamic results (ΔGoads and ∆Hoads).
The difference in EHOMO and ELUMO values, known as the energy gap (∆Eg) value, significantly influences the reactivity of the inhibitor molecule. As reported, the lower the ∆Eg value, the more significant the inhibitor molecule's reactivity, resulting in better adsorption at the metal surface [49]. The inhibitor's imino (5.106 eV) and protonated form (5.014 eV) shows lower ∆Eg values. It suggests that corrosion inhibition mainly occurs by the adsorption of imino and protonated ABT on the alloy surface.
The amino, imino, and protonated forms of ABT have planar geometry, resulting in a better inhibitor interaction with the alloy surface. In addition, parameters like hardness (η) and softness (σ) values are also crucial in evaluating the reactivity of the inhibitor molecules. Generally, a soft molecule shows higher reactivity than a hard molecule, because the former can easily donate electrons. As reported earlier, the inhibitor molecule with the highest σ value and lowest η value shows stronger adsorption and hence good inhibitive performance [50]. It is evident from Table 6 that both the imino and protonated forms of the inhibitor exhibit the highest softness but lowest hardness and ∆Eg value. Therefore, the imino and protonated forms may be responsible for the excellent inhibition performance shown by ABT.
The literature survey [51, 52] indicated that the ΔN value reveals the inhibition effect caused by electron donation. The fraction of electrons transferred from ABT molecules to the alloy surface was computed as per Eq. (17) [53]
where ηAl and ηInh indicate the hardness, while χAl and χInh represent the electronegativity of Al and inhibitor, respectively. In the present case, the theoretical value of electronegativity (ϕAl) is taken as 4.28 eV [54] and the hardness value for Al (ηAl) as zero [53]. The negative ΔN value for protonated inhibitor shows its higher electron-accepting trend. Lukovits and others reported that ΔN ˂3.6 indicate that the % IE value increases on increasing the electron-releasing tendency of the inhibitor molecules at the alloy surface [52].
The electrophilicity index (ω) value computed using Eq. (18) [55]
where µ denotes the electronic chemical potential, which is defined as [46]
The higher electron-donating tendency of the inhibitor's imino and amino forms is confirmed by their lowest electrophilicity index (ω) value. In comparison, the high electron-accepting nature of the protonated inhibitor may be due to its highest electrophilicity index value [56]. To conclude, amino, imino, and protonated forms equally contribute to the excellent inhibition performances shown by ABT.
3.9 A comparison of inhibition performance of ABT with reported inhibitors of AA6061
The inhibition performance of ABT is compared with other reported inhibitors of AA6061 alloys and summarized in Table 7. ABT showed a higher % IE than most reported inhibitors [13, 19, 57,58,59,60]. Some of the other reported inhibitors [61, 62] showed slightly higher % IE than ABT but at a very low concentration of HCl medium (0.01 and 0.1 M HCl). However, ABT exhibited a lower % IE than two other reported inhibitors [63, 64].
4 Conclusion
The following conclusions are drawn based on the investigation results:
-
The proposed inhibitor, ABT, was a potential inhibitor in 0.5 M HCl solution for AA6061-T6 corrosion control.
-
The inhibition efficiency of ABT improved with increased concentrations and a rise in medium temperature.
-
ABT displayed a mixed inhibitor behaviour towards AA6061-T6 in 0.5 M HCl solution.
-
The inhibition involves the mixed adsorption of ABT on the AA6061-T6 surface with the main chemisorption and follows the adsorption isotherm of Langmuir's.
-
The experimental results obtained by the PDP method verified by the EIS method.
-
The surface morphology and theoretical studies validated the experimental results of electrochemical methods.
References
Maisonnette D, Suery M, Nelias D, Chaudet P, Epicier T (2011) Effects of heat treatments on the microstructure and mechanical properties of a 6061 aluminium alloy. Mater Sci Eng A 528:2718–2724. https://doi.org/10.1016/j.msea.2010.12.011
Davis JR (1993) ASM specialty handbook: aluminium and aluminium alloys. ASM International, Metals Park
McCaffery E (2010) Introduction to corrosion science. Springer, New York
Murer N, Buchheit RG (2013) Stochastic modelling of pitting corrosion in aluminium alloys. Corros Sci 69:139–148. https://doi.org/10.1016/jcorsci.2012.11.034
Rozerfeld IL (1981) Corrosion inhibitors. McGraw Hill, New York
Xhanari K, Finšgar M (2016) Organic corrosion inhibitors for aluminium and its alloys in acid solutions: a review. RSC Adv. https://doi.org/10.1039/C6RA11818F
Shetty P (2020) Schiff bases: an overview of their corrosion inhibition activity in acid media against mild steel. Chem Eng Commun 207:985–1029. https://doi.org/10.1080/00986445.2019.1630387
Danaee I, Gholami M, Rashvand Avei M, Maddahy MH (2015) Quantum chemical and experimental investigations on inhibitory behavior of amino–imino tautomeric equilibrium of 2-aminobenzothiazole on steel corrosion in H2SO4 solution. J Ind Eng Chem 26:81–94. https://doi.org/10.1016/j.jiec.2014.11.018
Jafari H, Akbarzade K, Danaee I (2019) Corrosion inhibition of carbon steel immersed in a 1 M HCl solution using benzothiazole derivatives. Arab J Chem 12:1387–1394. https://doi.org/10.1016/j.arabjc.2014.11.018
Quraishi MA, Wajid Khan MA, Ajmal M, Muralidharan S, Venkatakrishna Iyer V (1996) Influence of substituted benzothiazoles on corrosion in acid solution. J Appl Electrochem 26:1253–1258. https://doi.org/10.1007/BF00249927
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Musa AY, Mohamad AB, Kadhum AAH, Chee EP (2011) Galvanic corrosion of aluminium alloy (Al2024) and copper in 1.0 M nitric acid. Int J Electrochem Sci 6:5052–5065
Nagalaxmi N, Shetty P, Kumari PP (2020) Inhibitive action of glutathione reduced on the deterioration of AA6061 in 0.5 M HCl. Tribol Ind 42:214–224. https://doi.org/10.24874/ti.785.10.19.02
Yurt A, Ulutas S, Dal H (2006) Electrochemical and theoretical investigation on the corrosion of aluminium in acidic solution containing some Schiff bases. Appl Surf Sci 253:919–925. https://doi.org/10.1016/j.apsusc.2006.01.026
Li WH, He Q, Zhang S, Pei S, Hou B (2007) Experimental and theoretical investigation of the adsorption behavior of new triazole derivatives as inhibitors for mild steel corrosion in acid media. Electrochim Acta 52:6386–6394. https://doi.org/10.1016/j.electacta.2007.04.077
Ahamed I, Quraishi MA (2009) Bis (benzimidazol-2-yl) disulphide: an efficient water soluble inhibitor for corrosion of mild steel in acid media. Corros Sci 51:2006–2013. https://doi.org/10.1016/j.corsci.2009.05.026
Noor EA (2009) Evaluation of inhibitive action of some quaternary N-heterocyclic compounds on the corrosion of Al–Cu alloy in hydrochloric acid. Mater Chem Phys 114:533–541. https://doi.org/10.1016/j.matchemphys.2008.09.065
Pinto GM, Nayak J, Shetty AN (2011) Corrosion inhibition of 6061 Al–15 vol pct SiC(p) composite and its base alloy in a mixture of sulphuric acid and hydrochloric acid by 4-(N, N-dimethyl amino)benzaldehyde thiosemicarbazone. Mater Chem Phys 125:628–640. https://doi.org/10.1016/j.matchemphys.2010.10.00
Kumari PP, Shetty P, Nagalaxmi DS (2020) Effect of cysteine as environmentally friendly inhibitor on AA6061-T6 corrosion in 0.5 M HCl: electrochemical and surface studies. Surf Eng Appl Electrochem 56:624–634. https://doi.org/10.3103/S1068375520050087
Metikos-Huković M, Babic R, Grubac Z (1998) Corrosion protection of aluminium in acidic chloride solutions with nontoxic inhibitors. J Appl Electrochem 28:433–439. https://doi.org/10.1023/A:1003200808093
Pinto GM, Nayak J, Shetty AN (2011) Adsorption and inhibitor action of 4-(N, N-dimethylamino) benzaldehyde thiosemicarbazone on 6061 Al SiC composite and its base alloy in sulfuric acid medium. Synth React Inorg Met Org Nanomet 41:37–41. https://doi.org/10.1080/15533174.2010.538023
Metikos-Hukovic M, Babic R, Grubac Z (2002) The study of aluminium corrosion in acidic solution with nontoxic inhibitors. J Appl Electrochem 32:35–41. https://doi.org/10.1023/A:1014265407060
Mansfeld F, Lin S, Kim S, Shih H (1988) Electrochemical impedance spectroscopy as a monitoring tool for passivation and localized corrosion of aluminium alloys. Mater Corros 39:487–492. https://doi.org/10.1002/maco.19880391102
Machnikova E, Kenton WH, Hckerman N (2008) Corrosion inhibition of carbon steel in hydrochloric acid by furan derivatives. Electrochim Acta 53:6024–6032. https://doi.org/10.1016/j.electacta.2008.03.021
Haque J, Verma C, Srivastava V, Quraishi MA, Ebenso EE (2018) Experimental and quantum chemical studies of functionalized tetrahydropyridines as corrosion inhibitors for mild steel in 1 M hydrochloric acid. Results Phys 9:1481–1493. https://doi.org/10.1016/j.rinp.2018.04.069
Kumari PP, Shetty P, Rao SA (2015) Corrosion protection properties of 4-hydroxy-N′-[(1E, 2E)-3-phenylprop-2-en-1-ylidene] benzo hydrazide on mild steel in hydrochloric acid medium. Prot Met Phys Chem 51:1034–1042. https://doi.org/10.1134/S2070205115060143
Bentiss F, Lebrini M, Lagrenee M (2005) Thermodynamic characterization of metal dissolution and inhibitor adsorption processes in mild steel/2,5-bis (n-thienyl)-1,3,4-thiadiazoles/hydrochloric acid system. Corros Sci 47:2915–2931. https://doi.org/10.1016/j.corsci.2005.05.034
Bessone JB, Salinas DR, Mayer CE, Ebert M, Lorenz W (1992) An EIS study of aluminium barrier-type oxide films formed in different media. Electrochim Acta 37:2283–2290. https://doi.org/10.1016/0013-4686(92)85124-4
Umoren SA, Obot IB, Ebenso EE, Okafor PC (2006) Gum arabic as a potential corrosion inhibitor for aluminium in alkaline medium and its adsorption characteristics. Anti-corros Methods Mater 53:277–282. https://doi.org/10.1108/00035590610692554
Szauer T, Brandt A (1981) On the role of fatty acid in adsorption and corrosion inhibition of iron by amine fatty acid salts in acidic solution. Electrochim Acta 26:1219–1224
Bentiss F, Bouanis M, Mernari B, Traisnel M, Vezin H, Lagrenee M (2007) Understanding the adsorption of 4H–1,2,4-triazole derivatives on mild steel surface in molar hydrochloric acid. Appl Surf Sci 253:3696–3704
Khaled KF (2008) New synthesized guanidine derivative as a green corrosion inhibitor for mild steel in acidic solutions. Int J Electrochem Sci 3:462–475
Behpour M, Ghoreishi SM, Soltani N, Salavati-Niasari M, Hamadanian M, Gandomi A (2008) Electrochemical and theoretical investigation on the corrosion inhibition of mild steel by thiosalicylaldehyde derivatives in hydrochloric acid solution. Corros Sci 50:2172–2181. https://doi.org/10.1016/j.corsci.2008.06.020
Verma C, Ebenso EE, Vishal Y, Quraishi MA (2016) Dendrimers: a new class of corrosion inhibitors for mild steel in 1 M HCl: experimental and quantum chemical studies. J Mol Liq 224:1282–1293. https://doi.org/10.1016/j.molliq.2016.10.117
Obot IB, Obi-Egbedi NO, Umoren SA (2009) Antifungal drugs as corrosion inhibitors for aluminium in 0.1 M HCl. Corros Sci 51:1868–1875. https://doi.org/10.1016/j.corsci.2009.05.017
Kliskic M, Radosevic J, Gudic S (2000) Katalinic V (2000) Aqueous extract of Rosmarinus officinalis L. as inhibitor of Al–Mg alloy corrosion in chloride solution. J Appl Electrochem 30:823–830. https://doi.org/10.1023/A1004041530105
Tang L, Li X, Si Y, Mu G, Liu G (2006) The synergistic inhibition between 8-hydroxy quinoline and chloride ion for the corrosion of cold rolled steel in 0.5 M sulfuric acid. Mater Chem Phys 95:29–38. https://doi.org/10.1016/j.matchemphys.2005.03.064
Amin MA, Ahmed MA, Arida HA, Arslan T, Saracoglu M, Kandemirli F (2011) Monitoring corrosion and corrosion control of iron in HCl by non-ionic surfactants of the TRITON-X series—Part II. Temperature effect, activation energies and thermodynamics of adsorption. Corros Sci 53:540–548. https://doi.org/10.1016/j.corsci.2010.09.019
Sayed Abd El Rehim S, Hamdi Hassan H, Mohammed Amin A (2003) The corrosion inhibition study of sodium dodecyl benzene sulphonate to aluminium and its alloys in 1.0 M HCl solution. Mater Chem Phys 78:337–348. https://doi.org/10.1016/S0254-0584(01)00602-2
Chidiebere MA, Ogukwe CE, Oguzie KL, Eneh CN, Oguzie EE (2012) Corrosion inhibition and adsorption behavior of Punica granatum extract on mild steel in acidic environments: experimental and theoretical studies. Ind Eng Chem Res 51:668–677. https://doi.org/10.1021/ie201941f
Bereket G, Pinarbsi A (2004) Electrochemical thermodynamic and kinetic studies of the behaviour of aluminium in hydrochloric acid containing various benzotriazole derivatives. Corros Eng Sci Technol 39:308–312. https://doi.org/10.1179/174327804X13136
Bereket G, Yurt A (2001) The inhibition effect of amino acids and hydroxyl carboxylic acids on pitting corrosion of aluminium alloy 7075. Corros Sci 43:1179–1195. https://doi.org/10.1016/S0010-938X(00)00135-
Gece G (2008) The use of quantum chemical methods in corrosion inhibitor studies. Corros Sci 50:2981–2922. https://doi.org/10.1016/j.corsci.2008.08.043
Yan X, Wu X, Xu J, Fan Y, Bi C, Zhang X, Zhang Z (2014) Crystal structure of 2-amino benzothiazolinium nitrate and theoretical study of the amino–imino tautomerism of 2-amino benzothiazole. Heterocycl Commun 20:167–174. https://doi.org/10.1515/hc-2014-0018
Tay F, Duran M, Demirayak S (2014) A quantum chemical DFT/HF study on acidity constants of some benzothiazole and thiozle derivatives. Indian J Chem 53B:102–110
Pearson RG (1988) Absolute electronegativity and hardness: application to inorganic chemistry. Inorg Chem 27:734–740
Chang-Guo Z, Nichols JA, Dixon DA (2003) Ionization potential, electron affinity, electronegativity, hardness, and electron excitation energy: molecular properties from density functional theory orbital energies. J Phys Chem A 107:4184–4195. https://doi.org/10.1021/jp0225774
Obot IB, Obi-Egbedi NO (2008) Fluconazole as an inhibitor for aluminium corrosion in 0.1 M HCl. Colloids Surf A 330:207–212. https://doi.org/10.1016/j.colsurfa.2008.07.058
Arslan T, Kandemirli F, Ebenso EE, Love I, Alemu H (2009) Quantum chemical studies on the corrosion inhibition of some sulphonamides on mild steel in acidic medium. Corros Sci 51:35–47. https://doi.org/10.1016/j.corsci.2008.10.016
Masoud MS, Awad MK, Shaker MA, El-Tahawy MMT (2010) The role of structural chemistry in the inhibitive performance of some amino pyrimidines on the corrosion of steel. Corros Sci 52:2387–2396. https://doi.org/10.1016/j.corsci.2010.04.011
Sastri VS, Elboujdaini M, Perumareddi JR (2005) Utility of quantum chemical parameters in the rationalization of corrosion inhibition efficiency of some organic inhibitors. Corrosion 61:933–942
Lukovits L, Kalman E, Zucchi F (2001) Corrosion inhibitors—correlation between electronic structure and efficiency. Corrosion 57:3–8. https://doi.org/10.5006/1.3290328
Pearson RG (1990) Hard and soft acids and bases—the evolution of a chemical concept. Coord Chem Rev 100(C):403–425
Kokalj A, Kovacevic N (2011) On the consistent use of electrophilicity index and HSAB-based electron transfer and its associated change of energy parameters. Chem Phys Lett 507:81–184. https://doi.org/10.1016/j.cplett.2011.03.045
Parr RG, Szentpaly LV, Liu S (1999) Electrophilicity index. J Am Chem Soc 121:1922–1924. https://doi.org/10.1021/ja983494x
Obot IB, Obi-Egbedi NO (2010) Adsorption properties and inhibition of mild steel corrosion in sulphuric acid solution by ketoconazole: experimental and theoretical investigation. Corros Sci 52:198–204. https://doi.org/10.1016/j.corsci.2009.09.002
Fakrudeen SP, Ananda Murthy HC, Bheema Raju V (2012) Corrosion inhibition of AA6061 and AA6063 alloy in hydrochloric acid media by Schiff base compounds. J Chil Chem Soc 57:1364–1371
Fakrudeen SP, Bheema Raju V (2013) Electrochemical behaviour of AA6061 alloy in 1 M hydrochloric acid using Schiff base compounds as corrosion inhibitors. J Mater Environ Sci 4:326–337
Charitha BP, Rao P (2015) Ecofriendly biopolymer as green inhibitor for corrosion control of 6061-aluminium alloy in hydrochloric acid medium. Int J ChemTech Res 8:330–342
Charitha BP, Rao P (2017) Electrochemical and adsorption studies for the corrosion control of 6061 Al alloy using eco-friendly inhibitor. Surf Eng Appl Electrochem 53:551–559. https://doi.org/10.3103/S1068375517060035
Raviprabha K, Bhat RS (2019) Inhibition effects of ethyl-2-amino-4-methyl-1, 3-thiazole-5-carboxylate on the corrosion of AA6061 alloy in hydrochloric acid media. J Fail Anal Prev 19:1464–1474. https://doi.org/10.1007/s11668-019-00744-5
Raviprabha K, Bhat RS (2019) 5-(3-Pryridyl)-4H-1, 2, 4-triazole-3-thiol as potential corrosion inhibitor for AA6061 aluminium alloy in 0.1 M hydrochloric acid solution. Surf Eng Appl Electrochem 55:723–733. https://doi.org/10.3103/S1068375519060103
Wang X, Huang A, Lin D, Talha M, Liu H, Lin Y (2020) Imidazolium-based ionic liquid as efficient corrosion inhibitor for AA 6061 alloy in HCl solution. Materials 13:4672. https://doi.org/10.3390/ma13204672
Maithili K, Shetty P, Kumari PP, Kagatikar S (2022) Mannich Base as an efficient corrosion inhibitor of AA6061 in 0.5 M HCl: electrochemical, surface morphological and theoretical investigations. Arab J Sci Eng 47:7053–7067. https://doi.org/10.1007/s13369-021-06302-2
Acknowledgements
All the authors thank the Manipal Institute of Technology, Manipal Academy of Higher Education, Manipal, for providing the necessary laboratory facilities.
Funding
Open access funding provided by Manipal Academy of Higher Education, Manipal. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Scaria, P.T., Shetty, P., Kumari, P.P. et al. 2-Aminobenzothiazole as an efficient corrosion inhibitor of AA6061-T6 in 0.5 M HCl medium: electrochemical, surface morphological, and theoretical study. J Appl Electrochem 52, 1675–1689 (2022). https://doi.org/10.1007/s10800-022-01742-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-022-01742-6