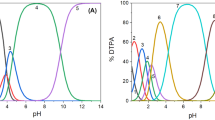
Abstract
Apart from the generally dominant trivalent oxidation state, several lanthanides can occur in divalent or tetravalent states as well. Changing the valence state alters the chemical properties and, therefore, gives the opportunity to ease intragroup lanthanide separations. The hydrated terbium(III) ion has a highly positive reduction potential (E0 = + 3.1 V vs. SHE), but it can be oxidized to its tetravalent state via ozonolysis or electrolysis, and stabilized in highly concentrated carbonate solutions. In this study, terbium(III) is electrochemically oxidized to terbium(IV) in aqueous carbonate, nitrate, and periodate media. Spectroscopic and elemental analysis were done to characterize terbium(IV) and to gain insight in the stability of these complexes in the aqueous electrolytes of carbonate, nitrate, and periodate. In all these electrolytes, oxidation and stabilization were achieved at different pH, terbium concentration, salt concentration and applied potentials. The most promising electrolyte was found to be carbonate, since it allowed to attain more concentrated and more stable terbium(IV) solutions.
Graphical abstract












Similar content being viewed by others

References
Binnemans K, Jones PT (2014) Perspectives for the recovery of rare earths from end-of-life fluorescent lamps. J Rare Earths 32:195–200. https://doi.org/10.1016/S1002-0721(14)60051-X
Van de Voorde M, Van Hecke K, Cardinaels T, Binnemans K (2019) Radiochemical processing of nuclear-reactor-produced radiolanthanides for medical applications. Coord Chem Rev 382:103–125. https://doi.org/10.1016/j.ccr.2018.11.007
Teo RD, Termini J, Gray HB (2016) Lanthanides: applications in cancer diagnosis and therapy. J Med Chem 59:6012–6024. https://doi.org/10.1021/acs.jmedchem.5b01975
Wang X, Chang H, Xie J et al (2014) Recent developments in lanthanide-based luminescent probes. Coord Chem Rev 273–274:201–212. https://doi.org/10.1016/j.ccr.2014.02.001
Cotton S (2006) Lanthanide and actinide chemistry and spectroscopy. John Wiley and Sons, New York, Uppingham, Rutland
Van de Voorde M, Van Hecke K, Binnemans K, Cardinaels T (2018) Separation of samarium and europium by solvent extraction with an undiluted quaternary ammonium ionic liquid: towards high-purity medical samarium-153. RSC Adv 8:20077–20086. https://doi.org/10.1039/C8RA03279C
Zuo Y, Liu Y, Chen J, Li DQ (2008) The separation of cerium(IV) from nitric acid solutions containing thorium(IV) and lanthanides (III) using pure [C8mim]PF6 as extracting phase. Ind Eng Chem Res 47:2349–2355. https://doi.org/10.1021/ie071486w
Kedari CS, Pandit SS, Ramanujam A (1997) In situ electro-oxidation and liquid-liquid extraction of cerium(IV) from nitric acid medium using tributyl phosphate and 2-ethylhexyl hydrogen 2-ethylhexyl phosphonate. J Radioanal Nucl Chem 222:141–147. https://doi.org/10.1007/BF02034260
Borai EH, Shahr El-Din AM, El Afifi EM, et al. (2016) Subsequent separation and selective extraction of thorium (IV), iron (III), zirconium (IV) and cerium (III) from aqueous sulfate medium. South African J Chem 69:148–156 https://doi.org/10.17159/0379-4350/2016/v69a18
Jelinek L, Wei Y, Arai T, Kumagai M (2007) Selective Eu(III) electro-reduction and subsequent separation of Eu(II) from rare earths(III) via HDEHP impregnated resin. Solvent Extr Ion Exch 25:503–513. https://doi.org/10.1080/07366290701415911
Jelinek L, Wei Y, Arai T, Kumagai M (2008) Study on separation of Eu(II) from trivalent rare earths via electro-reduction and ion exchange. J Alloys Compd 451:341–343. https://doi.org/10.1016/j.jallcom.2007.04.139
Van De Voorde M, Geboes B, Vander Hoogerstraete T et al (2019) Stability of europium(II) in aqueous nitrate solutions. Dalt Trans 48:14758–14768. https://doi.org/10.1039/c9dt03139a
Zelić M (2003) Electrochemical reduction of europium (3+) at increasing concentrations of different salts. Part I. Voltammetric measurements. Croat Chem Acta 76:241–248
Donohue T (1977) Photochemical separation of europium from lanthanide mixtures in aqueous solution. J Chem Phys 5402:10–13. https://doi.org/10.1063/1.434656
Qiu L-F, Kang X-H, Wang T-S (1991) A study on photochemical separation of rare earths: the separation of europium from an industrial concentrate material of samarium, europium, and gadolinium. Sep Sci Technol 26:199–221. https://doi.org/10.1080/01496399108050467
Myasoedov BF, Kulyako YM, Sklyarenko IS (1975) Electrochemical reduction of europium in acetonitrile. J Inorg Nucl Chem 37:113–117 https://doi.org/10.1016/0022-1902(75)80136-9
Rabockai T (1977) Electrochemical reduction of ytterbium in perchloric media. J Electroanal Chem Interfacial Electrochem 76:83–89 https://doi.org/10.1016/S0022-0728(77)80008-9
Rabockai T, Luis LO (1980) Electrochemical reduction of Eu3+ in aqueous ethylene glycol solutions. Electrochim Acta 25:1041–1042. https://doi.org/10.1016/0013-4686(80)87012-5
de Bulhões LO, Rabockai ST (1982) Electrochemical reduction of lanthanides in propylene carbonate. Electrochim Acta 27:1071–1073. https://doi.org/10.1016/0013-4686(82)80111-4
Steeman E, Temmerman E, Verbeek F (1978) Electrochemical reduction of the lanthanide ions: Part I. First reduction step of ytterbium(III) in acidic 1 M NaClO4 solution. J Electroanal Chem Interfacial Electrochem 89:97–111 https://doi.org/10.1016/S0022-0728(78)80035-7
Atanasyants AG, Seryogin AN (1995) The reaction of the electrochemical reduction Eu (III) + e- → Eu(II) in hydrochloric solution. Hydrometallurgy 37:367–374 https://doi.org/10.1016/0304-386X(94)00036-3
Zhao HL, You QC, Chun AM (2012) Electrochemical oxidation of Ce(III) to Ce(IV) in mixed acid (H2SO4 and CH3SO3H). Adv Mater Res 588–589:90–94. https://doi.org/10.4028/www.scientific.net/amr.588-589.90
Kiekens P, Steen L, Donche H, Temmerman E (1981) Kinetics of Ce(IV) reduction at gold, carbon and iridium electrodes. Electrochim Acta 26:841–845. https://doi.org/10.1016/0013-4686(81)85043-8
Gupta R, Gamare J, Jayachandran K et al (2015) Electrochemical, thermodynamic and spectroscopic investigations of CeIII in a 1-ethyl-3-methylimidazolium ethyl sulfate (EMIES) ionic liquid. Eur J Inorg Chem 2015:4396–4401. https://doi.org/10.1002/ejic.201500671
Knecht LA (1967) Oxidation of cerium(III) to cerium(IV) using a mixture of hot concentrated perchloric and sulfuric acids. Anal Chim Acta 39:349–355. https://doi.org/10.1016/S0003-2670(01)80514-6
Binnemans K (2006) Chapter 229 applications of tetravalent cerium compounds. In: Gschneidner KA, Bunzli J-CG, Pecharsky VKBT (eds) Handbook on the physics and chemistry of rare earths. Elsevier, pp. 281–392
Piro NA, Robinson JR, Walsh PJ, Schelter EJ (2014) The electrochemical behavior of cerium(III/IV) complexes: thermodynamics, kinetics and applications in synthesis. Coord Chem Rev 260:21–36. https://doi.org/10.1016/j.ccr.2013.08.034
Modiba P, Crouch AM (2008) Electrochemical study of cerium(IV) in the presence of ethylenediaminetetraacetic acid (EDTA) and diethylenetriaminepentaacetate (DTPA) ligands. J Appl Electrochem 38:1293–1299. https://doi.org/10.1007/s10800-008-9558-7
Gompa TP, Ramanathan A, Rice NT, La Pierre HS (2020) The chemical and physical properties of tetravalent lanthanides: Pr, Nd, Tb, and Dy. Dalt Trans 49:15945–15987. https://doi.org/10.1039/d0dt01400a
Frederick Smith G, Getz CA (1938) Cerate oxidimetry. Ind Eng Chem Anal Ed 10:191–195
Wadsworth E, Duke FR, Goetz CA (1957) Present status of cerium(IV)-cerium(III) potentials. Anal Chem 29:1824–1825
Wei H, Li Y, Zhang Z et al (2017) Selective extraction and separation of Ce (IV) and Th (IV) from RE(III) in sulfate medium using di(2-ethylhexyl)-N-heptylaminomethylphosphonate. Solvent Extr Ion Exch 35:117–129. https://doi.org/10.1080/07366299.2017.1292025
Sedneva TA (2005) Cerium(III) electrooxidation in nitric acid solutions of rare-earth elements. Russ J Appl Chem 78:907–911. https://doi.org/10.1007/s11167-005-0418-5
Yörükoğlu A, Girgin İ (2002) Recovery of europium by electrochemical reduction from sulfate solutions. Hydrometallurgy 63:85–91. https://doi.org/10.1016/S0304-386X(01)00215-8
Peppard DF, Horwitz EP, Mason GW (1962) Comparative liquid-liquid extraction behaviour of europium (II) and europium (III). J Inorg Nucl Chem 24:429–439. https://doi.org/10.1016/0022-1902(62)80039-6
Ying Y, Ru-Dong Y (1992) Synthesis and characterization of tetravalent terbium complexes of alkali terbium hexaoxidoiodates. Polyhedron 11:963–966. https://doi.org/10.1016/S0277-5387(00)83348-3
Josse M, Dubois M, El-Ghozzi M et al (2005) Anti-KSbF6 structure of CaTbF6 and CdTbF6: a confirmation of the singular crystal chemistry of Tb4+ in fluorides. Acta Crystallogr Sect B Struct Sci 61:1–10. https://doi.org/10.1107/S0108768104026928
Wang D, Qiu G, Jin X et al (2006) Electrochemical metallization of solid terbium oxide. Angew Chemie Int Ed 45:2384–2388. https://doi.org/10.1002/anie.200503571
Avignant D, Largeau E, Gaumet V et al (1998) Recent progress in tetravalent terbium chemistry. J Alloys Compd 275–277:1–5. https://doi.org/10.1016/S0925-8388(98)00262-X
Hobart DE (1981) Electrochemical and spectroscopic studies of some less stable oxidation states of selected lanthanide and actinide elements. Dissertation, University of Tennessee
Hobart DE, Samhoun K, Young JP et al (1980) Stabilization of praseodymium(IV) and terbium(IV) in aqueous carbonate solution. Inorg Nucl Chem Lett 16:321–328. https://doi.org/10.1016/0020-1650(80)80069-9
Propst RC (1974) Electrochemical oxidation of terbium(3) at the conducting glass electrode. J Inorg Nucl Chem 36:1085–1094. https://doi.org/10.1016/0022-1902(74)80218-6
Varlashkin PG, Begun GM, Peterson JR (1985) On the nature of tetravalent terbium in carbonate-hydroxide solutions. J Less Common Met 109:123–134. https://doi.org/10.1016/0022-5088(85)90112-2
Qiang S, Zhijian W, Chongli G (1995) Solvent extraction of terbium(III, IV) periodate complexes with quaternary amine. Solvent Extr Ion Exch 13:275–288. https://doi.org/10.1080/07366299508918274
Qiang S, Zhijian W, Chongli G (1996) Solvent extraction of tetravalent terbium Tb(IV) and other trivalent rare earths with quaternary amine. In: International solvent extraction conference. Melbourne, pp. 605–610
Li X, Dong W, Qi Y et al (1991) Studies on the stabilization of terbium(IV) in aqueous tetrametaphosphate solution. Polyhedron 10:1479–1483. https://doi.org/10.1016/S0277-5387(00)86069-6
Rice NT, Popov IA, Russo DR et al (2019) Design, isolation, and spectroscopic analysis of a tetravalent terbium complex. J Am Chem Soc 141:13222–13233. https://doi.org/10.1021/jacs.9b06622
Willauer AR, Palumbo CT, Scopelliti R et al (2020) Stabilization of the oxidation state +IV in siloxide-supported terbium compounds. Angew Chemie - Int Ed 59:3549–3553. https://doi.org/10.1002/anie.201914733
Palumbo CT, Zivkovic I, Scopelliti R, Mazzanti M (2019) Molecular complex of Tb in the +4 oxidation state. J Am Chem Soc 141:9827–9831. https://doi.org/10.1021/jacs.9b05337
Rao RR, Milliken SB, Robinson SL, Mann CK (1970) Anodic oxidation of lithium, cadmium, silver, and tetrabutylammonium nitrates. Anal Chem 42:1076–1080. https://doi.org/10.1021/ac60291a006
Mishima H, Iwasita T, Macagno VA, Giordano MC (1973) The electrochemical oxidation of nitrate ion on platinum from silver nitrate in acetonitrile solutions—I. kinetic analysis. Electrochim Acta 18:287–292. https://doi.org/10.1016/0013-4686(73)80030-1
Colombi M, Fiori G, Formaro L (1973) Electrochemical study of the anodic oxidation of nitrate ion on a platinum electrode in acetic acid. J Electroanal Chem Interfacial Electrochem 44:21–24. https://doi.org/10.1016/S0022-0728(73)80510-8
Moeller T (1953) Inorganic chemistry. John Wiley and Sons, New York
Acknowledgements
This research was funded by Research Foundation – Flanders (FWO) – SBO project Terbium Isotopes for Medical Applications in Flanders (Tb – IRMA – V, Grant number S005019N).
Funding
This study was funded by fonds wetenschappelijk onderzoek (S005019N).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Arman, M.O., Geboes, B., Van Hecke, K. et al. Electrochemical oxidation of terbium(III) in aqueous media: influence of supporting electrolyte on oxidation potential and stability. J Appl Electrochem 52, 583–593 (2022). https://doi.org/10.1007/s10800-021-01651-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-021-01651-0