Abstract
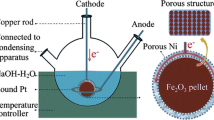
Electrolysis of iron ore is a promising CO2-free iron production route based on the electrolytic reduction of solid iron oxide particles suspended in 110 °C concentrated alkaline electrolyte. The reactivities of different iron compounds during their reduction into iron through this process have been compared using a model laboratory cell. Chronoamperometry experiments were performed on suspensions containing hematite (α-Fe2O3), magnetite (Fe3O4) or goethite (α-FeOOH) at a cell voltage of 1.66 V. Current density response, anode and cathode electrochemical potentials, faradaic efficiency and iron deposit morphology were compared. Hematite reduces to iron at 1100 A/m2 with current yield near 85%. For goethite, the current density response was 33% lower and current efficiency dropped by 20% compared to hematite. Magnetite reactivity proved to be extremely low with eight-time lower current density and tenfold lower current efficiency than hematite. The weaker reactivity of goethite and magnetite particles could be ascribed to their more difficult adsorption on the cathode surface partly covered with metal iron, the far higher viscosity of goethite suspensions, although at the same solid concentrations as the other oxide particles and the occurrence of opposite electrode reactions with dissolved Fe(OH)3 − and Fe(OH)4 − ions.
Graphical Abstract








Similar content being viewed by others
References
Picard G, Oster D, Tremillon B (1980) Electrochemical reduction of iron oxides in suspension in water-sodium hydroxide mixtures between 25 and 140 °C. Part II. Experimental study. J Chem Res (S) 8:252–253
Allanore A, Lavelaine H, Valentin G, Birat JP, Lapicque F (2007) Electrodeposition of metal iron from dissolved species in alkaline media. J Electrochem Soc 154(12):E187-E193
Allanore A, Lavelaine H, Valentin G, Birat JP, Lapicque F (2008) Iron metal production by bulk electrolysis of iron ore particles in aqueous media. J Electrochem Soc 155(9):125–129
Allanore A, Lavelaine H, Valentin G, Birat JP, Delcroix P, Lapicque F (2010) Observation and modeling of the reduction of hematite particles to metal in alkaline solution by electrolysis. Electrochim Acta 55(12):4007–4013
Siebentritt M, Volovitch P, Ogle K, Lefèvre G (2014) Adsorption and electroreduction of hematite particles on steel in strong alkaline media. Colloids Surf A 440:197–201
Duchateau A (2013) Réduction par électrolyse de nanoparticules d’oxydes de fer en milieu alcalin à 110 °C. Dissertation, University ParisTech
Monteiro JF, Ivanova YA, Kovalevsky AV, Ivanou DK, Frade JR (2016) Reduction of magnetite to metallic iron in strong alkaline medium. Electrochim Acta 193:284–292
Gorbunova KM, Liamina LI (1966) On the mechanism of iron reduction from alkaline solutions. Electrochim Acta 11:457–467
Blakey BC, James DF (2003) The viscous behaviour and structure of aqueous suspensions of goethite. Colloids Surf A 231(1–3):19–30
Nickell RA, Zhu WH, Payne RU, Cahela DR, Tatarchuk BJ (2006) Hg/HgO electrode and hydrogen evolution potentials in aqueous sodium hydroxide. J Power Sources 161(2):1217–1224
Zou X, Lu GSX, Xie X, Lu C, Zhou Z, Ding W (2015) Electroreduction of iron(III) oxide pellets to iron in alkaline media: a typical shrinking-core reaction process. Metall Mater Trans B 46(3):1262–1274
Balej J (1985) Determination of the oxygen and hydrogen overvoltage in concentrated alkali hydroxide solutions. Int J Hydrogen Energy 10(6):365–374
Tarasevitch MR, Daskowski A, Yeager E (1983) Oxygen electrochemistry. In: Conway BE, Bockris JO’M, Yeager E, Khan SUM (eds) Comprehensive treatise of electrochemistry 7. Plenum Press, New York
Wendt H, Plzak V (1983) Electrocatalytic and thermal activation of anodic oxygen-and cathodic hydrogen-evolution in alkaline water electrolysis. Electrochim Acta 28(1):27–34
Yuan B, Kongstein OE, Haarberg GM (2009) Electrowinning of iron in aqueous alkaline solution using a rotating cathode. J Electrochem Soc 156(2):D64-D69
Wagner W, Pruß A (2002) The IAPWS formulation 1995 for the thermodynamic properties of ordinary water substance for general and scientific use. J Phys Chem Ref Data 31(2):387–535
Barin I (2008) Thermochemical data of pure substances, 3rd edn. Wiley, New York
Balej J (1996) Activity coefficients of aqueous solutions of NaOH and KOH in wide concentration and temperature ranges. Coll Czechoslov Chem Comm 61:1549–1562
Acknowledgements
Thanks are due to ANRT for financial support in V. Feynerol’s PhD grant. The work has been partly funded by Ademe in the Valorco project (2014–2018).
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
The reactive adsorption reactions considered in this paper involve several solid compounds and liquid water in a 50 wt% NaOH–H2O mixture. Gibbs free enthalpy of any of these reactions was obtained by summing the chemical potential of every compound involved in the balance reaction multiplied by their algebraic stoichiometric coefficient.
Each solid compound was considered a pure compound, therefore their chemical potential is equal to their standard chemical potential at considered temperature under 1 bar, that is to say to their molar Gibbs free energy at considered temperature under 1 bar:
Thermodynamic data used were IAPWS-95 [16] model for water thermodynamic properties and Barin’s data [17] for the heat capacities of solid compounds.
The chemical potential of water can be expressed as follows:
IAPWS-95 model was used to represent pure water Gibbs free energy. In order to calculate pure liquid water Gibbs free energy at a temperature above standard boiling point, pressure was increased to water saturated vapour pressure at considered temperature. At 110 °C, the saturated vapour pressure calculated by IAPWS-95 model is 143 309 Pa, i.e., approx. 1.43 bar. For such a low pressure difference, thermodynamic properties of liquid phase will barely change.
Akerlof and Kegeles semi-empirical model which has more recently described by Balej [18], was used to represent water activity. The data used for this model were for temperature lower than 70 °C and molality lower than 17 mol/kg, hence the equation of water activity was extrapolated to the conditions of this study. For the conditions of interest, the water activity was estimated to be near 0.15.
Rights and permissions
About this article
Cite this article
Feynerol, V., Lavelaine, H., Marlier, P. et al. Reactivity of suspended iron oxide particles in low temperature alkaline electrolysis. J Appl Electrochem 47, 1339–1350 (2017). https://doi.org/10.1007/s10800-017-1127-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-017-1127-5