Abstract
In this work, we report a facile and one -step hydrothermal method to decorate SnO2 nanostructures on few-layered graphene for superior dopamine detection. The structural and morphological properties of the prepared nanohybrids illustrated the tetragonal crystal system of SnO2, reconstruction of graphene layers with oxygen containing functional groups, increased ID/IG ratio, and uniform decoration of SnO2 nanostructures on graphene layers. The prepared nanohybrids exhibited a high electrochemical activity toward dopamine oxidation in comparison with individual graphene sheets. This enhanced performance can be due to the presence of SnO2 nanostructures between the graphene layers, which efficiently avoid the restacking and increase the surface area accessibility. The fabricated nanohybrid-based sensor showed an increase in current with respect to the increased analyte concentration over the wide range of 0.005–0.20 × 10−6 M. The sensor exhibits excellent catalytic activity toward dopamine with the lowest detection limit of 6.3 nm. Further, the modified electrode exhibited good stability, reproducibility, and better recovery of 99 % in human urine samples suggesting the real-time usability of the sensor.
Graphical Abstract
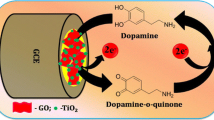
Schematic representation of SnO2 nanohybrids formation. The modified electrode exhibits superior catalytic activity. The oxidation peak current increased linearly in the concentration range of 0.005–0.019 × 10−6 M with LOD of 6.3 nm.









Similar content being viewed by others
References
Novoselov KS, Geim AK, Morozov SV, Jiang D, Zhang Y, Dubonos SV (2004) Electric field effect in atomically thin carbon films. Science 306:666–669. doi:10.1126/science.1102896
Zhu J, Chen M, He Q, Shao L, Wei S, Guo Z (2013) An overview of the engineered graphene nanostructures and nanocomposites. RSC Adv 3:22790. doi:10.1039/c3ra44621b
Khan M, Tahir MN, Adil SF, Khan HU, Siddiqui MRH, Al-warthan AA et al (2015) Graphene based metal and metal oxide nanocomposites: synthesis, properties and their applications. J Mater Chem A 3:18753–18808. doi:10.1039/C5TA02240A
Galvan A, Wichmann T (2008) Pathophysiology of Parkinsonism. Clin Neurophysiol 119:1459–1474. doi:10.1016/j.clinph.2008.03.017
Wightman RM, May LJ, Michael AC (1988) Detection of dopamine dynamics in the brain. Anal Chem 60:769A–779A
Jackowska K, Krysinski P (2012) New trends in the electrochemical sensing of dopamine. Anal Bioanal Chem 405:3753–3771. doi:10.1007/s00216-012-6578-2
Yang L, Liu D, Huang J, You T (2014) Simultaneous determination of dopamine, ascorbic acid and uric acid at electrochemically reduced graphene oxide modified electrode. Sensors Actuators B Chem 193:166–172. doi:10.1016/j.snb.2013.11.104
Pandikumar A, Soon How GT, See TP, Omar FS, Jayabal S, Kamali KZ et al (2014) Graphene and its nanocomposite material based electrochemical sensor platform for dopamine. RSC Adv 4:63296–63323. doi:10.1039/C4RA13777A
Pingarrn JM, Sedeo PY, Gonzlez-Corts A (2008) Gold nanoparticle-based electrochemical biosensors. Electrochim Acta 53:5848–5866. doi:10.1016/j.electacta.2008.03.005
Kuang Q, Lao C, Wang ZL, Xie Z, Zheng L (2007) High-sensitivity humidity sensor based on a single SnO2 nanowire. J Am Chem Soc 129:6070–6071. doi:10.1021/ja070788m
Kaniyoor A, Baby TT, Ramaprabhu S (2010) Graphene synthesis via hydrogen induced low temperature exfoliation of graphite oxide. J Mater Chem 20:8467–8469
Pullamsetty A, Subbiah M, Sundara R (2015) Platinum on boron doped graphene as cathode electrocatalyst for proton exchange membrane fuel cells. Int J Hydrogen Energy 40:10251–10261. doi:10.1016/j.ijhydene.2015.06.020
Zhang M, Lei D, Du Z, Yin X, Chen L, Li Q et al (2011) Fast synthesis of SnO2/graphene composites by reducing graphene oxide with stannous ions. J Mater Chem 21:1673–1676. doi:10.1039/C0JM03410J
Ferrari AC, Robertson J (2000) Interpretation of Raman spectra of disordered and amorphous carbon. Phys Rev B 61:14095–14107. doi:10.1103/PhysRevB.61.14095
Sripada R, Bhagavathi Parambath V, Baro M, Nagappan Nair SP, Sundara R (2015) Platinum and platinum–iron alloy nanoparticles dispersed nitrogen-doped graphene as high performance room temperature hydrogen sensor. Int J Hydrogen Energy 40:10346–10353. doi:10.1016/j.ijhydene.2015.06.018
Wang H, Maiyalagan T, Wang X (2012) Review on recent progress in nitrogen-doped graphene: synthesis, characterization, and its potential applications. ACS Catal 2:781–794. doi:10.1021/cs200652y
Liang J, Wei W, Zhong D, Yang Q, Li L, Guo L (2012) One-step in situ synthesis of SnO2/graphene nanocomposites and its application as an anode material for Li-ion batteries. ACS Appl Mater Interfaces 4:454–459. doi:10.1021/am201541s
Yu KN, Xiong Y, Liu Y, Xiong C (1997) Microstructural change of nano- SnO2 grain assemblages with the annealing temperature. Phys Rev B 55:2666–2671. doi:10.1103/PhysRevB.55.2666
Seema H, Christian Kemp K, Chandra V, Kim KS (2012) Graphene-SnO2 composites for highly efficient photocatalytic degradation of methylene blue under sunlight. Nanotechnology 23:355705. doi:10.1088/0957-4484/23/35/355705
Gu F, Wang SF, Song CF, Qi YX, Zhou GJ et al (2003) Synthesis and luminescence properties of SnO2 nanoparticles. Chem Phys Lett 372:451–454. doi:10.1016/S0009-2614(03)00440-8
Baraneedharan P, Hussain SI, Dinesh VP, Siva C, Biji P, Sivakumar M (2015) Lattice doped Zn–SnO2 nanospheres: a systematic exploration of dopant ion effects on structural, optical, and enhanced gas sensing properties. Appl Surf Sci 357:1511–1521. doi:10.1016/j.apsusc.2015.09.257
Ma HF, Chen TT, Luo Y, Kong FY, Fan DH, Fang HL, Wang W (2015) Electrochemical determination of dopamine using octahedral SnO2 nanocrystals bound to reduced reduced graphene oxide nanosheets. Microchim Acta 182:2001–2007
Chen D, Feng H, Li J (2012) Graphene oxide: preparation, functionalization, and electrochemical applications. Chem Rev 112:6027–6053. doi:10.1021/cr300115g
Sajid M, Nazal MK, Mansha M, Alsharaa A, Jillani SMS, Basheer C (2016) Chemically modified electrodes for electrochemical detection of dopamine in the presence of uric acid and ascorbic acid: a review TrAC—Trends. Anal Chem 76:15–29. doi:10.1016/j.trac.2015.09.006
Zhang H, He Q, Zhu X, Pan D, Deng X, Jiao Z (2012) Surfactant-free solution phase synthesis of monodispersed SnO2 hierarchical nanostructures and gas sensing properties. Cryst Eng Comm 14:3169. doi:10.1039/c2ce06558d
Sun W, Wang X, Wang Y, Ju X, Xu L, Li G et al (2013) Application of graphene-SnO2 nanocomposite modified electrode for the sensitive electrochemical detection of dopamine. Electrochim Acta 87:317–322. doi:10.1016/j.electacta.2012.09.050
Yang K, Xue Y, Zhang Y, Zhang XF, Zhao H, Li XJ et al (2013) A simple one-pot synthesis of graphene nanosheet/SnO2 nanoparticle hybrid nanocomposites and their application for selective and sensitive electrochemical detection of dopamine. J Mater Chem B 1:1804–1811. doi:10.1039/C3tb00513e
Zhang F, Li Y, Gu Y-e, Wang Z, Wang C (2011) One-pot solvothermal synthesis of a Cu2O/graphene nanocomposite and its application in an electrochemical sensor for dopamine. Microchim Acta 173:103–109. doi:10.1007/s00604-010-0535-6
Yang J, Strickler JR, Gunasekaran S (2012) Indium tin oxide-coated glass modified with reduced graphene oxide sheets and gold nanoparticles as disposable working electrodes for dopamine sensing in meat samples. Nanoscale 4:4594. doi:10.1039/c2nr30618b
Sun CL, Lee HH, Yang JM, Wu CC (2011) The simultaneous electrochemical detection of ascorbic acid, dopamine, and uric acid using graphene/size-selected Pt nanocomposites. Biosens Bioelectron 26:3450–3455. doi:10.1016/j.bios.2011.01.023
Acknowledgments
The authors thank SAIF, IIT Madras for providing the facility of HR-SEM and help in FTIR measurements. One of the authors, Dr. PB thanks CSIR-HRDG for providing support under the Scientist Pool Scheme (No.13(8778-A)/2015-Pool) and conveys heartfelt thanks to his lab colleagues for their support in this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baraneedharan, P., Alexander, S. & Ramaprabhu, S. One-step in situ hydrothermal preparation of graphene–SnO2 nanohybrid for superior dopamine detection. J Appl Electrochem 46, 1187–1197 (2016). https://doi.org/10.1007/s10800-016-1001-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-016-1001-x