Abstract
Purpose
To assess the effectiveness of a switch to faricimab in individuals affected by DME and previously treated with aflibercept.
Methods
In this retrospective, single-center study, DME patients previously treated with at least 3 injections of aflibercept then switched to faricimab were enrolled. Best corrected visual acuity (BCVA) and central subfield thickness (CST) were recorded at baseline, at the time of the switch and at 6 months follow-up. At transition to faricimab, patients were categorized as "good visual responders" (≥ 5 letters from baseline) or "poor visual responders" (< 5 letters), and as "good anatomical responders" (any reduction in edema compared to baseline) or "poor anatomical responders" (no reduction or worsening of edema). Changes in BCVA and CST were recorded at 6 months after the switch to faricimab.
Results
100 eyes of 100 patients (61 female, 61%) were switched to faricimab after a mean of 6.8 ± 3.3 aflibercept injections. At the 6 months follow-up, only “poor visual responders” (N = 62) demonstrated a meaningful increase in BCVA (Δswitch-6M = + 5 letters; P = 0.007), coupled with a reduction in CST (Δswitch-6M = − 67.9 µm; P = 0.004); participants with “poor anatomical response” upon transitioning exhibited a significant functional gain (Δswitch-6M = + 4.5 letters; p = 0.05) but limited CST enhancements (Δswitch-6M = − 95.1 µm; p = 0.05).
Conclusions
Faricimab shows a positive impact on anatomical and functional metrics in DME cases refractory to aflibercept.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic macular edema (DME) continues to significantly contribute to vision impairment and blindness worldwide in individuals who have diabetes. Presently, clinical trials with solid level 1 evidence have indicated that agents targeting antivascular endothelial growth factor (VEGF), namely ranibizumab and aflibercept, along with off-label bevacizumab, are the most efficacious therapeutic choices for enhancing visual acuity and macular structure in cases of center-involved DME when compared to laser treatment [1,2,3]. The trials RISE-RIDE for ranibizumab, VIVID-VISTA for aflibercept, and DRCR Retina Protocol T for bevacizumab have illustrated that nearly 40% of patients witnessed an improvement of 15 or more letters on Snellen eye charts after 2 years of follow-up [1,2,3,4,5].
Nevertheless, a subset of patients exhibit suboptimal responses to anti-VEGF therapy, and approximately 30% of DME patients continue to experience persistent DME even after a year of adequate treatment [6, 7]. Additionally, the number of injections administered in clinical practice is generally fewer than those in clinical trials, leading to suboptimal visual outcomes [9].
Therefore, it seems reasonable to consider transitioning between different anti-VEGF agents if the initial treatment fails to address macular edema sufficiently. Several small-scale studies have shown positive changes in both anatomical and functional aspects when DME patients switch from ranibizumab or bevacizumab to aflibercept [10,11,12,13], albeit with relatively short follow-up periods ranging from 1 to 6 months. On the contrary, other small studies suggest an improvement solely in anatomical aspects, with no significant alteration in visual function upon switching to aflibercept [14,15,16,17].
Recently, the emergence of faricimab, a bispecific antibody targeting both angiopoietin-2 (Ang-2) and VEGF-A, has revitalized prospects for enhanced treatment approaches in DME [18]. By concurrently inhibiting two distinct pathways involved in angiogenesis, faricimab offers the potential for a more robust and sustained response, thereby reducing treatment frequency and enhancing the quality of life for DME patients [19].
In theory, faricimab could exhibit enhanced efficacy in cases of DME that exhibit inadequate responsiveness to aflibercept, attributed to its capacity for Ang-2 inhibition [18]. Consequently, this study aimed to assess the effectiveness of a shift to faricimab in individuals affected by DME and previously treated with aflibercept.
Materials and methods
This study is a retrospective single-center investigation. The data analyzed consisted of consecutive patients with diabetic macular edema (DME) who transitioned from receiving intravitreal injections of aflibercept to faricimab injections at the Eye Institute of Cleveland Clinic Abu Dhabi. The study period ranged from August 2022 to July 2023. The study was approved by the Ethics Committee of Cleveland Clinic Abu Dhabi, adhered to the principles outlined in the Declaration of Helsinki regarding research involving human subjects.
The inclusion criteria were as follows: (1) age of 18 years or older; (2) diagnosis of either type 1 or type 2 diabetes mellitus (DM); (3) clinically significant, center-involving DME according to EDTRS guidelines; (4) best-corrected visual acuity (BCVA) ranging from 20/200 to 20/20; (5) central subfield thickness (CST) equal to or greater than 300 μm, as measured by spectral domain optical coherence tomography (SD-OCT); (6) a minimum of 3 previous aflibercept intravitreal injections; (7) a minimum follow-up period of 6 months after the anti-VEGF switch.
The reason for switching was an effort to reduce the number of intravitreal injections.
The exclusion criteria were: (1) macular edema caused by factors other than diabetic retinopathy (e.g., retinal vein occlusion, age-related macular degeneration, postsurgical macular edema); (2) vitreo-retinal interface disorder such as vitreo-macular traction and epiretinal membrane; (3) significant media opacity that impeded the quality of OCT imaging (e.g., corneal opacity, cataract, vitreous hemorrhage); (4) history of ocular trauma or surgery within 6 months prior to the first faricimab injection; (5) prior administration of intravitreal corticosteroids before faricimab injection; (6) intravitreal anti-vascular endothelial growth factor (anti-VEGF) treatment (bevacizumab, ranibizumab, or aflibercept) within 1 month prior to the faricimab injection; (7) uncontrolled glaucoma, defined as intraocular pressure (IOP) exceeding 25 mm Hg despite the use of antiglaucoma medication in the study eye.
We collected demographic information (age and gender), hemoglobin A1c (HbA1c) levels, and the duration of DME for each patient. Best-corrected visual acuity was measured using standard ETDRS charts at three time points: at baseline diagnosis of DME prior to aflibercept intravitreal treatment, at the time when the patients switched from aflibercept intravitreal injections to faricimab injections, and at the 6 months follow-up post switch. At the time of the transition to faricimab, patients were categorized as either "good visual responders" (with an improvement of ≥ 5 letters from baseline) or "poor visual responders" (with an improvement of < 5 letters).
The two functional and the two anatomical groups were analyzed s in a post hoc analysis.
Throughout the study period, all patients underwent a comprehensive ophthalmic evaluation, which included slit-lamp biomicroscopy, applanation tonometry, fundus biomicroscopy, and SD-OCT (Spectralis HRA, Heidelberg Engineering, Heidelberg, Germany). The CST was measured at baseline, at the time of the switch to faricimab, and at the 6 months follow-up. Based on the CST measurements at the time of the switch, patients were classified as either "good anatomical responders" (showing any reduction in edema compared to baseline) or "poor anatomical responders" (showing no reduction or worsening of edema compared to baseline).
When patients were switched to faricimab, the decision was to treat using an as-needed regimen (which followed an OCT-guided treatment protocol).
The primary endpoint was the mean variation in BCVA between the pre-switch time and month 6 after the switch.
Statistical analysis
Statistical analysis including descriptive statistics for demographics and main clinical records, and comparative analysis (Student’s t-test analysis for independent and paired samples and one-way analysis of Variance with Bonferroni correction) were performed through the open access R software (R Studio Version 1.1.383, R Project, www.r-project.org).
Continuous variables were reported as the median and interquartile range, while categorical variables were presented as frequencies and percentages. A P value of 0.05 or less was considered statistically significant. If a case had missing data for any of the variables, it was excluded from the analysis. Main outcome of the study was to evaluate if there is a significant clinical benefit of switching patients on existing anti-VEGF therapy to Faricimab.
Results
Overall, 100 eyes of 100 patients (61 female, 61%), mean age 60 ± 9 years, were included in the analysis and followed for 8.4 ± 1.8 months after switching. Twelve patients were affected by type 1 DM (12%) and mean HbA1c was 8.4% ± 1.6%. Baseline clinical characteristics, stratified on the functional and anatomical outcome at anti-VEGF switch, did not vary among groups.
At switch to faricimab, data sub-analysis based on visual and anatomical response performed after a mean of 6.8 ± 3.3 aflibercept injections over a period of 11.3 ± 6.8 months, revealed that 62 eyes (62%) had a “poor visual response” (− 4.3 ± 10.7 letters), while 38 eyes (38%) disclosed “good visual response” (+ 13.9 ± 9.2 letters). Stratifying patients on the basis of OCT changes, 50 eyes (50%) showed no edema lowering or edema worsening, while 50 eyes (50%) showed any CST improvement. Patients with poor visual response were significantly associated with more limited morphological improvement (Fisher’s exact test for categorical variables P = 0.03).
Mean number of faricimab injections over the follow-up period of 6 months was 2.9 ± 0.9.
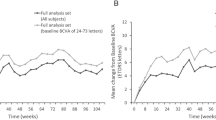
Globally, within the study cohort, there was an observed but statistically non-significant decline in visual function (Δswitch-6M = − 0.2; P = 0.04, Student’s t-test for paired samples; Fig. 1A) alongside a notable enhancement in anatomic structure on OCT (Δswitch-6M = − 77.4 μm; P < 0.001, Student’s t-test for paired samples; Fig. 1B) following a 6-month transition to faricimab.
When stratified by best-corrected visual acuity (BCVA) outcomes, only individuals with suboptimal visual responses demonstrated a meaningful increase in BCVA subsequent to the switch to faricimab injections (59.6 ± 18 letters, Δswitch-6M = + 5; p = 0.007, Student’s t-test for paired samples; Fig. 1A), coupled with a reduction in CST (331 ± µm, Δswitch-6M = − 67.9 µm; p = 0.004, Student’s t-test for paired samples; Fig. 1B). Conversely, those initially exhibiting favorable visual responses to aflibercept showed only marginal BCVA changes in response to faricimab (59.8 ± 21 letters, Δswitch-6M = + 2; p = 0.2, Student’s t-test for paired samples; Fig. 1A), despite a substantial improvement in morphological parameters (345.1 ± µm, Δswitch-12M = − 82 µm; p = 0.002, Student’s t-test for paired samples; Fig. 1B) (Table 1).
Analyzing the cohorts according to changes in CST, participants with no initial reduction or even an increase in CST upon transitioning exhibited a greater functional gain (60.75 ± 13 letters, Δswitch-6M = + 4.5; p = 0.05, Student’s t-test for paired samples;) in comparison to those manifesting any degree of edema reduction after aflibercept treatment (60.75 ± 9 letters, Δswitch-6M = + 1.7; p = 0.3, Student’s t-test for paired samples) (Fig. 1A). In terms of anatomical response (Fig. 1B), individuals with suboptimal anatomical improvements displayed limited CST enhancements with the use of faricimab (376.8 ± µm, Δswitch-6M = − 95.1 µm; p = 0.05, Student’s t-test for paired samples), whereas those with any edema reduction after transitioning showed a more substantial CST improvement (305.5 ± 87 µm, Δswitch-6M = − 59.1 µm; p = 0.07, Student’s t-test for paired samples) (Table 1).
Discussion
In the present study we analyzed the functional and anatomical response in DME patients after switching from aflibercept to faricimab, and found that only patients with a poor visual and anatomical response to aflibercept benefit from the switch in terms of BCVA and CST. Patients who were responding to aflibercept either functionally or anatomically, do not exhibit a statistically significant increase in BCVA after the switch to faricimab.
Although the utilization of anti-VEGF injections has exhibited improvements in both the structural and functional outcomes for patients afflicted with DME, a subset of individuals display less-than-optimal responses [6, 7]. While the principal of switching remains controversial, when clinicians encounter such situations, they often face the decision of switching therapy to either intravitreal corticosteroids or attempting an alternative anti-VEGF agent. Given its comparably lower propensity for adverse effects, particularly among younger patients, the adoption of an alternative anti-VEGF agent is often deemed more favorable over corticosteroids.
A limited number of investigations have evaluated the results of transitioning to aflibercept following prolonged anti-VEGF treatment for persistent DME. Lim et al. [20] documented noteworthy enhancements in both functional and structural aspects after switching to aflibercept in 21 eyes from 19 DME patients who had demonstrated unsatisfactory responses to numerous bevacizumab/ranibizumab injections. The approach to aflibercept treatment post-transition varied in this study, with a median of 3 injections over an average 5-month follow-up and a 2.4-month interval between each aflibercept injection. Bahrami et al. [21] similarly showcased the favorable impact of aflibercept on visual and morphological improvements among DME patients with suboptimal responses to prior bevacizumab injections. Wood et al. [15], however, exclusively observed morphological enhancements with aflibercept among patients with inadequate responses to ranibizumab and/or bevacizumab injections, albeit the majority (11 of 14) were evaluated following only a single aflibercept injection. Rahimy et al. [22] similarly noted a mere morphological response to aflibercept injections subsequent to previous bevacizumab/ranibizumab therapy, attributing this outcome to irreversible functional damage stemming from prolonged DME.
Notably, the transition to aflibercept resulted in anatomical amelioration for the majority of patients across all these studies. Aflibercept possesses notably greater affinity for VEGF-A compared to bevacizumab or ranibizumab, and it additionally binds with VEGF-B and placental growth factor (PGF) [23]. The latter is a cytokine that can stimulate angiogenesis and plays a crucial role in the activation and maintenance of the inflammatory switch associated with neo-angiogenesis. Placental growth factor has been implicated in the pathogenesis of diabetic retinopathy and DME [24].
Based on previous findings, it has been observed that about 30% of patients exhibit DME resistant to effective treatment for a year [6, 7]. Even following 3 years of regular aflibercept treatment, 13% of DME patients continue to necessitate frequent dosing [25].
Faricimab, a bispecific antibody that simultaneously inhibits Vascular Endothelial Growth Factor (VEGF) and Angiopoietin 2 (ANG2), has been proposed as a novel approach to treat diabetic macular edema [19].
The role of VEGF in the pathogenesis of multiple blinding eye diseases has been well established in the literature. Despite its integral role in preserving the vascular equilibrium across various cellular structures and tissue types under normal physiological conditions, it has been implicated in the molecular etiology and pathogenesis of retinopathies associated with a range of ocular pathologies, including age-related macular degeneration (AMD), diabetic retinopathy (DR), as well as diabetic macular edema (DME). Therefore, therapeutic interventions that inhibit VEGF and its related pathways have been instrumental in averting visual impairment in a significant population of ocular disease patients, especially diabetic macular edema. Another potential therapeutic target is the cytokine ANG2, which is implicated in both angiogenesis and immune response modulation, thereby presenting as a promising candidate for the management of exudative AMD and other retinal pathologies. Observations of elevated intraocular ANG2 concentrations in patients with diabetic retinopathy and retinal vein occlusion underscore the potential clinical relevance of ocular ANG2 inhibition [19].
Faricimab, a novel bispecific antibody developed by Roche/Genentech, targets both VEGF-A and ANG2. It was approved by the FDA in 2022 for the treatment of wet AMD (w-AMD) and DME after meeting primary endpoints in phase III clinical trials, namely YOSEMITE and RHINE [19]. Both trials were randomized multi-center studies. In the YOSEMITE study, 73.8% of patients, and in the RHINE study, 71.1% of patients, experienced similar improvements in BCVA with faricimab compared to aflibercept. Notably, this was achieved with fewer injections [19, 28]. Furthermore, the anatomical response was also positive—reduction in intra-retinal fluid and central subfield thickness was more significant in the Faricimab arm of both trials. In line with earlier research, Faricimab demonstrated an overall good tolerability, and the occurrence of adverse events was comparable across the groups under study.
These findings suggest that faricimab may improve the visual and anatomical outcomes of patients with fewer injections than are necessary using the previous anti-VEGF injections.
In the present study, aflibercept-resistant DME patients who after a mean of 6.8 injections did not achieve a functional improvement (N = 62) benefited from a switch to faricimab injections both in terms of BCVA (+ 5 letters gained at 6 months, P = 0.007) and of CST (− 67.9 µm CST reduction at 6 months, P = 0.004). In addition, 50 patients who were not responding to aflibercept injections in terms of CST reduction, achieved a statistically significant functional result at 6 months after switching to faricimab (+ 4.5 letters gained at 6 months, P = 0.05).
The greater increase in BCVA and reduction in macular thickness in the non-responders patients who were switched might be explained by the blocking of all isoforms of VEGF along with blocking of ANG2. However, this improvement in visual acuity with faricimab may also be related to patients' inherent characteristics rather than features of faricimab. In addition to all these possible explanations, patients treated with repetitive aflibercept injections may demonstrate tachyphylaxis or a diminished therapeutic response to this agent over time as suggested in a great number of studies [26].
To our knowledge, very few previous study investigated the effectiveness of Faricimab in treating anti-VEGF-resistant DME [27]. Rush and Rush [28] investigated this aspect via a retrospective review of DME patients who had been receiving Aflibercept therapy. Subjects were divided into a study group which was switched to faricimab as well as a control group which continued with aflibercept. Their results revealed that a significant subset of the study group (37.5%) achieved a CST of less than 300 µm without retinal fluid on OCT after the 4-month study period, compared to 3.7% in the control group. Moreover, 41.7% of the study group experienced an improvement of two or more lines of visual acuity, in contrast to 11.1% in the control group. Both findings were statistically significant. The study concluded that faricimab can improve the short-term visual and anatomic outcomes in treatment-resistant DME patients formerly managed with aflibercept.
On the other hand, our results showed that DME patients who were responding to aflibercept injections either functionally (N = 38) or anatomically (N = 50), did not benefit from the switch to faricimab (respectively + 2 and + 1.7 letters gained at 6 months). This goes along with recent findings from Protocol T showing that selected patients with suboptimal anti-VEGF response at the 12-week mark exhibited improved BCVA at the 2-year point without altering their anti-VEGF agents, suggesting a delayed positive response in certain cases [29]. This may suggest that some DME cases are predominantly driven by the VEGF pathway such that regardless of the agent used, when VEGF blockade is achieved, a good clinical outcome results.
Our study's scope is confined by its retrospective design. The decisions regarding treatment and the frequency of visits or treatments were contingent upon the preferences of individual ophthalmologists, potentially introducing bias in the selection process for faricimab injections, and varying the number of previous aflibercept injections prior to the treatment shift, which ranged from 3 to 10.
Moreover, the absence of a control group for patient comparison leaves room for the possibility that sustained aflibercept treatment, regardless of initial response, could have led to gradual enhancements in both CST and BCVA over an extended period.
Further limitations are attributed to the relatively brief follow-up period, potentially impacting our ability to thoroughly assess the enduring effectiveness of faricimab. Notably, faricimab's introduction in the UAE in May 2022, with its prescription at Cleveland Clinic Abu Dhabi commencing in September 2022, resulted in a limited pool of eligible patients due to the short timeframe for faricimab administration. While the 6-month follow-up was not designed for long-term treatment assessment, it aimed to validate the efficacy of faricimab treatment in cases where other therapies for DME had proven unsuccessful.
Additionally, the duration of DME, treatment history, count of anti-VEGF injections, and intervals between visits and injections varied across participants, precluding a direct head-to-head comparison between faricimab's efficacy and that of prior anti-VEGF therapies.
Notwithstanding the inherent limitations of this study, we present real-world data with the longest follow-up period to date, suggesting a consistently positive impact on anatomical and functional outcomes when transitioning from aflibercept to faricimab for poorly responding DME cases. At the same time, patients with a good visual and anatomical response to aflibercept should continue treatment as there seems to be no benefit in switching anti-VEGF agent. However, larger scale studies with longer follow-up and a control arm are imperative to pinpoint the specific patient subgroup that may benefit from treatment switching, along with the optimal timing for switching.
Data availability
No datasets were generated or analysed during the current study.
References
Nguyen QD, Brown DM, Marcus DM et al (2012) RISE and RIDE Research Group. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 119(4):789–801
Brown DM, Schmidt-Erfurth U, Do DV et al (2015) Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology 122(10):2044–2052
Rajendram R, Fraser-Bell S, Kaines A et al (2012) A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Arch Ophthalmol 130(8):972–979
Wu L, Martínez-Castellanos MA, Quiroz-Mercado H et al (2008) Twelve-month safety of intravitreal injections of bevacizumab (Avastin): results of the Pan-American Collaborative Retina Study Group (PACORES). Graefes Arch Clin Exp Ophthalmol 246(1):81–87
Diabetic Retinopathy Clinical Research Network, Scott IU, Edwards AR, Beck RW et al (2007) A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology 114(10):1860–1867
Wells JA, Glassman AR, Ayala AR et al (2015) Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 372:1193–1203
Bressler NM, Beaulieu WT, Glassman AR et al (2018) Diabetic retinopathy clinical research network. Persistent macular thickening following intravitreous aflibercept, bevacizumab, or ranibizumab for central-involved diabetic macular edema with vision impairment: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol 136(3):257–269
Ciulla TA, Pollack JS, Williams DF (2021) Visual acuity outcomes and anti-VEGF therapy intensity in diabetic macular oedema: a real-world analysis of 28 658 patient eyes. Br J Ophthalmol 105(2):216–221
Shimura M, Kitano S, Muramatsu D et al (2020) Japan Clinical Retina Study (J-CREST) group. Real-world management of treatment-naïve diabetic macular oedema in Japan: two-year visual outcomes with and without anti-VEGF therapy in the STREAT-DME study. Br J Ophthalmol 104(9):1209–1215
Mira F, Paulo M, Henriques F, Figueira J (2017) Switch to aflibercept in diabetic macular edema patients unresponsive to previous anti-VEGF therapy. J Ophthalmol 2017:5632634
Klein KA, Cleary TS, Reichel E (2017) Effect of intravitreal aflibercept on recalcitrant diabetic macular edema. Int J Retina Vitreous 3(3):16
Chen YY, Chang PY, Wang JK (2017) Intravitreal aflibercept for patients with diabetic macular edema refractory to bevacizumab or ranibizumab: analysis of response to aflibercept. Asia-Pac J Ophthalmol 6(3):250–255
Konidaris VE, Tsaousis KT, Al-Hubeshy Z, Pieri K, Deane J, Empeslidis T (2017) Clinical real-world results of switching treatment from ranibizumab to aflibercept in patients with diabetic macular oedema. Eye (Lond) 31(11):1629–1630
Demircan A, Alkin Z, Yesilkaya C, Demir G, Kemer B (2018) Comparison of intravitreal aflibercept and ranibizumab following initial treatment with ranibizumab in persistent diabetic macular edema. J Ophthalmol 19:4171628
Wood EH, Karth PA, Moshfeghi DM, Leng T (2015) Short-term outcomes of aflibercept therapy for diabetic macular edema in patients with incomplete response to ranibizumab and/or bevacizumab. Ophthalmic Surg Lasers Imaging Retina 46(9):950–954
Shah CP, Heier JS (2016) Aflibercept for diabetic macular edema in eyes previously treated with ranibizumab and/or bevacizumab may further improve macular thickness. Ophthalmic Surg Lasers Imaging Retina 47(9):836–839
Rahimy E, Shahlaee A, Khan MA, Ying G-S, Maguire JI, Ho AC et al (2016) Conversion to aflibercept after prior anti-VEGF therapy for persistent diabetic macular edema. Am J Ophthalmol 164:118-127.e2
Sahni J, Patel SS, Dugel PU et al (2019) Simultaneous inhibition of angiopoietin-2 and vascular endothelial growth factor-a with faricimab in diabetic macular edema: BOULEVARD phase 2 randomized trial. Ophthalmology 126(8):1155–1170
Wykoff C, Abreu F, Adamis AP et al (2022) Efficacy, durability, and safety of intravitreal faricimab with extended dosing up to every 16 weeks in patients with diabetic macular oedema (YOSEMITE and RHINE): two randomised, double-masked, phase 3 trials. Lancet 399:741–755
Lim RH, Gupta B, Simcock P (2017) Intravitreal aflibercept in neovascular age-related macular degeneration previously treated with ranibizumab. Int J Ophthalmol 10(3):423–426
Bahrami B, Hong T, Zhu M, Schlub TE, Chang A (2017) Switching therapy from bevacizumab to aflibercept for the management of persistent diabetic macular edema. Graefes Arch Clin Exp Ophthalmol 255(6):1133–1140
Rahimy E, Shahlaee A, Khan MA et al (2016) Conversion to aflibercept after prior anti-VEGF therapy for persistent diabetic macular edema. Am J Ophthalmol 164:118–27.e2
Papadopoulos N, Martin J, Ruan Q et al (2012) Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis 15(2):171–185
Nguyen QD, De Falco S, Behar-Cohen F et al (2018) Placental growth factor and its potential role in diabetic retinopathy and other ocular neovascular diseases. Acta Ophthalmol 96(1):e1–e9
Wykoff CC, Ou WC, Khurana RN, Brown DM, Lloyd Clark W, Boyer DS (2018) Long-term outcomes with as-needed aflibercept in diabetic macular oedema: 2-year outcomes of the ENDURANCE extension study. Br J Ophthalmol 102(5):631–636
Forooghian F, Cukras C, Meyerle CB, Chew EY, Wong WT (2009) Tachyphylaxis after intravitreal bevacizumab for exudative age-related macular degeneration. Retina 29(6):723–731
Durrani AF, Momenaei B, Wakabayashi T et al (2023) Conversion to faricimab after prior anti-vascular endothelial growth factor therapy for persistent diabetic macular oedema. Br J Ophthalmol 12:bjo-2023-324394. https://doi.org/10.1136/bjo-2023-324394
Rush RB, Rush SW (2022) Faricimab for treatment-resistant diabetic macular edema. Clin Ophthalmol 24(16):2797–2801
Bressler NM, Beaulieu WT, Maguire MG et al (2018) Early response to anti-vascular endothelial growth factor and two-year outcomes among eyes with diabetic macular edema in protocol T. Am J Ophthalmol 195:93–100
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation was performed by FP, IEG and AA, data collection by AA and SA, and analysis by FP and AA. The first draft of the manuscript was written by FP, AA and NG, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pichi, F., Abdi, A., Aljneibi, S. et al. Switch to faricimab after initial treatment with aflibercept in eyes with diabetic macular edema. Int Ophthalmol 44, 275 (2024). https://doi.org/10.1007/s10792-024-03226-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10792-024-03226-2