Abstract
Purpose
To evaluate the effect of pregnancy on the anterior chamber, corneal parameter, and intraocular pressure measurements; and compare the results between trimesters, postpartum and non-pregnant healthy age-matched women.
Methods
This prospective study included 41 pregnant women and 53 non-pregnant women. Four measurements were taken from the pregnant women, in each trimester and postpartum third month, and once from the control group. Of the individuals included in the study, anterior chamber depth (ACD), anterior chamber volume (ACV), K1 (flat keratometry), K2 (steep keratometry), Kmean (mean value of K1 and K2), anterior chamber angle (ACA), central corneal thickness (CCT), thinnest corneal thickness (TCT), astigmatism value (AST), corneal volume (CV), biometry, axial length (AL), spherical equivalent (SFEQ), intraocular lens power (ILP), VA (visual acuity) datas were recorded.
Results
We observed a statistically significant decrease in K2, CCT, ACD, AL and CV in the postpartum period (p = 0.025, p < 0.001, p = 0.029, p = 0.005, p = 0.004 respectively) and a statistically significant increase in ACV, CCT, and TCT as the gestational week progressed in the pregnant group (p = 0.007, p < 0.001, p = 0.025, respectively). A statistically significant decrease in IOP towards to the third trimester, and an increase in the postpartum period was observed (p < 0.001). We did not observe statistically significant changes in K1, Kmean, AST, ACA, VA, ILP, and SFEQ values.
Conclusion
It is important to investigate the physiological changes that may occur during pregnancy, distinguish them from pathological changes, and avoid unnecessary treatment. We consider that it’s also important to guide the timing of anterior segment surgeries such as cataract and refractive surgery and to prescribe glasses/contact lenses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pregnancy is a physiological process that affects many systems such as cardiovascular, pulmonary, renal, hematological, and visual systems, especially the endocrine system [1]. Hormonal, coagulative, and hemodynamic changes are responsible for most of the ocular adaptations [2]. In previous studies, changes between trimesters were observed in anterior segment parameters in pregnant women. These changes are a decrease in intraocular pressure (IOP) [3], a decrease in corneal sensitivity [4], an increase in corneal curvature [5] and a decrease in central corneal thickness [6]. Although the etiology is not known, it is returned to first trimester values at the end of the breastfeeding period; therefore, it has been suggested that it may occur due to corneal edema due to hormonal changes [7].
Anterior segment topographies are currently used to evaluate anterior segment parameters. Using Pentecam Scheimplug technology (Pentecam Oculus Optikgeräte GmbH, Wetzlar, Germany) gives us detailed information about anterior segment parameters [8]. Since it is a noncontact and noninvasive method, it is extremely safe to use in special patient groups such as pregnant women.
There is a limited number of studies evaluating anterior segment parameters in pregnant women. In these studies, measurements of different pregnant subjects were compared while evaluating the measurements between trimesters [9, 10]. We think that the anterior segment parameter values obtained from different individuals may affect the results of the studies due to individual differences [10]. Measurements taken from the same individuals increase the reliability of the results, and there is no study in the literature in which the same individuals are followed in each trimester of pregnancy. Therefore, in this study, we measured a total of 4 measurements, in the first, second, third, and postpartum periods, from subjects who were followed up from the beginning of pregnancy and compared them with the healthy age- and sex-matched control group.
Material method
This prospective case–control study was approved by the local ethics committee of the Akdeniz University Faculty of Medicine (Approval Number KAEK-841) and the study was conducted in compliance with the ethical standards set out in the Declaration of Helsinki. Informed consent was obtained from the participants who agreed to participate in the study.
All subjects underwent a comprehensive ophthalmological examination, including measurements of refractive error (Nidek ARK-700A, Nidek Co., Ltd, Gamagori, Japan), best-corrected visual acuity (BCVA), IOP /Full Auto Tonometer, NIDEK NT-2000, Nidek Co., Ltd., Aichi, Japan), biometry and axial length (AL), (IOLMASTER 500, Carl Zeiss Meditec AG, Jena, Germany), slit-lamp examination of the anterior and posterior segment, and Scheimflug Topography (Pentecam Oculus Optikgeräte GmbH, Wetzlar, Germany). The BCVA was converted into the logarithm of minimal angle resolution (logMAR).
Of the individuals included in the study, anterior chamber depth (ACD), anterior chamber volume (ACV), K1 (flat keratometry), K2 (steep keratometry), Kmean (mean value of K1 and K2), anterior chamber angle (ACA), central corneal thickness (CCT), thinnest corneal thickness (TCT), astigmatism value (AST), corneal volume (CV), axial length (AL), spherical equivalent (SFEQ), intraocular lens power (ILP), and VA (visual acuity) data were recorded.
Pregnant women were followed for about 1 year, in the first, second, third trimesters, and postpartum third months, and four measurements were taken from the same pregnant subjects in total. One measurement was taken from the control group. All measurements were made by the same ophthalmologist (ÇEP) between 14:00 and 16:00 to avoid diurnal variation. All participants had no pathology in their right eyes, and the right eyes of the participants were included in the study. The study began with 55 patients from both groups. 14 patients from the pregnant group were excluded from the study because they did not continue the follow-up, and 2 patients from the control group were excluded because their image quality was not good.
Inclusion criteria for the pregnant group Uncompleted singleton pregnancies, who are at a gestational age less than 14 weeks in the first examination and continue their follow-up, and who do not have a systemic disease related to pregnancy (gestational diabetes, preeclampsia/eclampsia, etc.).
Inclusion criteria for the control group Nonpregnant healthy women not in menopause and not taking hormone replacement therapy.
Inclusion criteria for both groups Between 20 and 40 years of age, without any systemic disease (such as diabetes mellitus, hypertension, thyroid disease), not using systemic and/or ocular drugs, with a spherical refractive error of less than 4 diopters (D) and/or cylindrical refractive errors of less than 2 D. Individuals without previous ocular surgery and ocular pathology affecting visual acuity and anterior segment parameters were included in the study.
Statistical analysis
Statistical analyzes were performed using the IBM SPSS package, version 23.0 (SPSS Inc., Chicago, IL, USA). The Shapiro–Wilk test was used to analyze the normality of sample distribution. To define the sample, normally distributed values are presented as means ± standard deviation, and non-normally distributed values are presented as median (minimum–maximum). The two-way repeated measures ANOVA test was used to examine the time-dependent change of the data. For the analysis of independent data, the independent-sample t-test and Mann–Whitney U test were used. For the analysis of correlation, Spearman’s correlation coefficient was used. The results were evaluated at a 95% CI. A level of p < 0.05 was accepted as statistically significant.
Results
A total of 94 eyes, including 41 pregnant women and 53 eyes of non-pregnant healthy age- and sex-matched controls, were included in the study. The demographic features of the subjects are summarized in Table 1. No statistically significant difference was observed between the mean ages of the subjects in the groups. (p = 0.217) (Table 1). Mean values of all anterior segment parameters in control, first, second, and third trimesters and postpartum groups and p values are shown in Table 1 and Table 2.
There was a statistically significant difference between trimesters and K1 values in the pregnancy group; there was no statistically significant difference between the postpartum period and the control group (p = 0.076, p = 0.301, respectively). We found a statistically significant increase in K2 value in the second trimester compared to the first trimester in the pregnancy group (p = 0.038), but we did not observe a statistically significant difference when the three trimesters were evaluated (p = 0.137). We found the K2 values during pregnancy to be statistically significantly higher than the postpartum values (p = 0.025). We did not find a statistically significant difference in corneal astigmatism values between trimesters and when compared with the postpartum and control groups (p = 0.066, p = 0.111, p = 0.978, respectively) (Table 2).
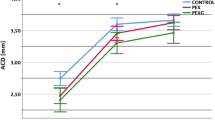
When trimesters were compared, we observed a statistically significant increase in TCT and CCT values between trimesters in the pregnant subjects (p = 0.025, p < 0.001, respectively). We observed a statistically significant increase in ACV values as the gestational week progressed (p = 0.007) (Table 2). ACV values are shown in Fig. 1.
We found the CV measurements to be higher in the pregnancy group than the postpartum measurements (p = 0.004). When the trimesters were compared among themselves, we found a statistically significant increase in the 3rd trimester compared to the 2nd trimester (p = 0.025). There was a significant decrease in IOP between trimesters and compared to the postpartum period and control group (p < 0.001, p < 0.001, p = 0.002, respectively) (Table 1). The IOP change is shown in Fig. 2.
When we examined the correlation between the gestational week and the measured values, there was a negative correlation between IOP and gestational week (p = 0.002, r = − 0.275). We observed a positive correlation between gestational week in ACD (p = 0.008, r = 0.299). We did not find a statistically significant correlation between other parameters and gestational week.
Discussion
In our study, we found a statistically significant decrease in K2, CCT, ACD, AL and CV in the postpartum period and a statistically significant increase in ACV, CCT, and TCT as the gestational week progressed in the pregnant group. We found a statistically significant decrease in IOP towards to the third trimester, and an increase in the postpartum period. We did not find statistically significant changes in K1, Kmean, AST, ACA, VA, ILP, and SFEQ values.
During pregnancy, many organs, including the eye and orbit, are affected by the physiological interaction between the mother and the fetus. These changes are more prominent, especially in the third trimester and regress in the postpartum period [11,12,13]. It was thought that the steepening of the corneal curvature would increase the refractive power of the cornea, but this effect would be slightly compensated due to the existing corneal edema [7, 14]. In our study, we found that myopia increased towards the last trimester and decreased in the postpartum period. This is in agreement with Pizzarello’s study, they found a change in favor of myopia during pregnancy and it returned to pre-pregnancy in the postpartum period [15]. This is in contrary to Ataş et al. refractive values were similar during the third trimester and the postpartum period [13].
In our study, we found that AL increased during pregnancy, but this increase was not statistically significant. However, in pairwise comparisons, it increased in the third trimester compared to the first trimester (p = 0.042) and decreases in postpartum measurements. This is in agreement with Sen et al. they evaluated the same parameters with the same device (IOL Master 500 Carl Zeiss Meditec Inc.. Jena. Germany) [16].
In our study, we did not observe a statistically significant difference between biometry values and intraocular lens power measurement during pregnancy and in the postpartum period. There was no difference between the control group and the pregnant group, and we did not observe any significant changes in visual acuity during pregnancy. This is in agreement with Manchester and Taner et al. no accommodative disorders or visual impaierment were found in the examination of pregnant women [7, 17].
In our study, we found that the K1 value increased towards the third trimester, however, the difference was not statistically significant (p = 0.09). Furthermore, there was no statistically significant change in K2 value during pregnancy. This is in agreement with Efe, Manchester and Taradaj et al. observed no significant alterations in keratometric parameters due to pregnancy [6, 10, 12]. This is in contrary to Park. and Manchester et el. observed an increase in corneal curvature during the second and third trimesters which resolved postpartum or after the cessation of breastfeeding [5, 7]. Although the etiology of this steepening of the cornea is not clear, it has been suggested that it may occur due to corneal edema caused by hormonal changes [7]. Based on this information, it was recommended not to prescribe glasses and contact lenses to pregnant women because of variable keratometry [7]. We think that the differences in K values in previous studies might be due to the comparison of different pregnant subjects for each trimester.
Many studies have been conducted on IOP changes during pregnancy [18, 19]. Most studies found a decrease in IOP during pregnancy [20]. The mechanism of IOP decrease was not exactly clarified. It is considered that increased levels of estrogen, relaxin, progesterone, and β-human chorionic gonadotropin amplify the outflow of aqueous humor through the unconventional track. [6, 12, 13, 21]. Since the reduction in systemic vascular resistance causes a reduction in episcleral venous pressure causes a reduction in IOP is another possible factor [18]. We found a 12% IOP decrease in third trimester of pregnancy and intraocular pressure returned to first trimester values in the postpartum period and the decrease in intraocular pressure increased as the gestational week progressed. This is in agreement with Tolunay, Özkaya, Efe, and Weinreb et al. who reported that IOP decreased in the second and the third trimesters compared to the first trimester [6, 10, 22, 23].
There are limited studies in the literature evaluating ACV, ACD, and ACA measurements in pregnancies. Changes in anterior chamber parameters are expected due to increased aqueous humor outflow and fluid retention in the body during pregnancy [13]. In our study, we observed a statistically significant increase in ACV in the second trimester and a decrease in the postpartum period. We did not detect any changes in ACA values. This is in agreement with Ataş et al. ACV values were significantly higher during the third trimester compared with the third month postpartum [13]. This is in agreement with Goldich et al. they observed no significant differences in the ACD, or ACA between pregnant women in their third trimester and nonpregnant women [21]. However in contrary to our study, Goldich et al. did not detect any difference in ACV values [21]. To the best of our knowledge, our study is the first study in which these parameters were evaluated prospectively during pregnancy trimesters and the postpartum period. Further study series with larger numbers of patients are needed to further comment on changes in the anterior chamber.
Weinreb et al. suggested that fluid retention during pregnancy can lead to higher corneal thickness [23]. This situation was previously explained by the inclusion of androgen, estrogen, and progesterone receptors in the human cornea [11, 24, 25]. In our study, we found a significant increase in CCT in the third trimester, and we found the corneal thickness decreased in the postpartum period. This is in agreement with Efe et al. the mean CCT in the second and third trimesters of pregnancy was measured to be higher than in the first trimester and at 3 months postpartum period [6]. Similarly, this is in agreement with Atas et al. demonstrated an increase in corneal thickness in the third trimester of pregnancy compared to the postpartum period [13]. The results of prospective studies were similar to our study. Errors that may arise from individual differences could be eliminated with measurements taken from the same individuals [26].
In addition to corneal thickness, several previous studies have evaluated corneal volume (CV). In our study although the increase in corneal volume was detected in the last trimester was not statistically significant; the decrease in the postpartum period was statistically significant (p = 0.04). We observed the corneal volume to be significantly higher starting from the first trimester compared to the postpartum period. This could show that the increase in corneal volume begins in the early stages of pregnancy and returns to normal after delivery. This is in contrary to Goldich et al. no difference was found between the third trimester pregnant and control groups [21]. This is in agreement with Ataş et el. CV was higher during the third trimester of pregnancy in comparison with the third month postpartum [13].
This study has some limitations. First, the sample size is small. Second, although the menstrual cycle can affect corneal biomechanical parameters we couldn’t take measurements from non-pregnant and postpartum women at the same menstrual cycle stage. Another limitation is that no measurements were made after the cessation of breastfeeding. Larger studies with larger sample groups are needed.
In conclusion, we found a statistically significant increase in ACV, CCT, and TCT towards the third trimester in the pregnant group, and a statistically significant decrease in K2, CCT, ACD, AL and CV in the postpartum period. We found a statistically significant decrease in IOP towards the third trimester and a statistically significant increase in the postpartum period. We think that it is important to investigate the physiological changes that may occur during pregnancy, to distinguish it from pathological changes, to avoid unnecessary treatment, to prescribe glasses/contact lenses, and to guide the timing of anterior segment surgeries such as cataract and refractive surgery.
Data availability
All data and materials are available from the supplementary material.
References
Sunness JS (1988) The pregnant woman’s eye. Surv Ophthalmol 32(4):219–238
Schocket LS, Grunwald JE, Tsang AF, DuPont J (1999) The effect of pregnancy on retinal hemodynamics in diabetic versus nondiabetic mothers. Am J Ophthalmol 128(4):477–484. https://doi.org/10.1016/s0002-9394(99)00234-2
Akar Y, Yucel I, Akar ME, Zorlu G, Ari ES (2005) Effect of pregnancy on intraobserver and intertechnique agreement in intraocular pressure. Ophthalmologica. https://doi.org/10.1159/000081781
Riss B, Riss P (1981) Corneal sensitivity in pregnancy. Ophthalmologica 183:57–62. https://doi.org/10.1159/000309139
Park SB, Lindahl KJ, Temnycky GO, Aquavella JV (1992) The effect of pregnancy on corneal curvature. CLAO J 18(4):256–259
Efe YK, Ugurbas SC, Alpay A, Ugurbas SH (2012) The course of corneal and intraocular pressure changes during pregnancy. Can J of Ophthalmol 47(2):150–154
Manchester PT Jr (1970) Hydration of the cornea. Trans Am Ophthalmol Soc 68:425–461
Motlagh MN, Moshirfar M, Murri MS, Skanchy DF, Momeni-Moghaddam H, Ronquillo YC (2019) Pentacam® corneal tomography for screening of refractive surgery candidates: a review of the literature, part ı. Med Hypothesis Discov Innov Ophthalmol. 8(3):177–203
Teberik K, Başbuğ A, Sağlam H, Karaarslan M, Kaya M (2018) Evaluation of anterior segment parameters and retinal nerve fiber layer thickness according to pregnancy trimester. Konuralp Med J 10(2):213–217
Özkaya D, Usta G, Karaca U, Özkaya MO (2022) Evaluation of anterior segment parameters during pregnancy. Semin ophthalmol 37(2):131–135. https://doi.org/10.1080/08820538.2021.1896748
Gupta PD, Johar K Sr, Nagpal K, Vasavada AR (2005) Sex hormone receptors in the human eye. Surv Ophthalmol 50(3):274–284
Taradaj K, Ginda T, Ciechanowicz P, Maciejewicz P, Suchońska B, Szymusik I, Kociszewska-Najman B (2018) Changes in the parameters of the anterior segment of the eye in pregnant women—literature review. Ginekol Pol 89(3):169–173. https://doi.org/10.5603/GP.a2018.0028
Ataş M, Duru N, Ulusoy DM, Altınkaynak H, Duru Z, Açmaz G (2014) Evaluation of anterior segment parameters during and after pregnancy. Cont Lens Anterior Eye 37(6):447–450. https://doi.org/10.1016/j.clae.2014.07.013
Schaal KB, Munk MR, Wyssmueller I, Berger LE, Zinkernagel MS, Wolf S (2019) Vascular abnormalities in diabetic retinopathy assessed with swept-source optical coherence tomography angiography widefıeld ımagıng. Retin 39(1):79–87. https://doi.org/10.1097/IAE.0000000000001938
Pizzarello LD (2003) Refractive changes in pregnancy. Graefes Arch Clin Exp Ophthalmol 241(6):484–488
Sen E, Onaran Y, Nalcacioglu-Yuksekkaya P, Elgin U, Ozturk F (2014) Corneal biomechanical parameters during pregnancy. Eur J Ophthalmol 24(3):314–319. https://doi.org/10.5301/ejo.5000378
Taner P, Akarsu C (2001) Gebeliğin Göz Üzerindeki Etkileri. J Retina-Vitreous 9(2):169–178
Green K, Phillips CI, Cheeks L, Slagle T (1988) Aqueous humor flow rate and intraocular pressure during and after pregnancy. Ophthalmic Res 20(6):353–357. https://doi.org/10.1159/000266751
Avasthi P, Sethi P, Mithal S (1976) Effect of pregnancy and labor on intraocular pressure. Int Surg 61(2):82–84
Qureshi IA (1996) Intraocular pressure and pregnancy: a comparison between normal and ocular hypertensive subjects. Arch Med Res 28(3):397–400
Goldich Y, Barkana Y, Pras E, Fish A, Mandel Y, Hirsh A (2011) Variations in corneal biomechanical parameters and central corneal thickness during the menstrual cycle. J Cataract Refract Surg 37(8):1507–1511. https://doi.org/10.1016/j.jcrs.2011.03.038
Tolunay HE, Özcan SC, Şükür YE, Özarslan Özcan D, Adıbelli FM, Hilali NG (2016) Changes of intraocular pressure in different trimesters of pregnancy among Syrian refugees in Turkey: a cross-sectional study. Turk J Obstet Gynecol 13(2):67–70. https://doi.org/10.4274/tjod.40221
Weinreb RN, Lu A, Beeson C (1988) Maternal corneal thickness during pregnancy. Am J Ophthalmol 105(3):258–260. https://doi.org/10.1016/0002-9394(88)90006-2
Suzuki T, Kinoshita Y, Tachibana M, Matsushima Y, Kobayashi Y, Adachi W (2001) Expression of sex steroid hormone receptors in human cornea. Curr Eye Res 22(1):28–33. https://doi.org/10.1076/ceyr.22.1.28.6980
Wickham LA, Gao J, Toda I, Rocha EM, Ono M, Sullivan DA (2000) Identification of androgen, estrogen and progesterone receptor mRNAs in the eye. Acta Ophthalmol Scand 78(2):146–153. https://doi.org/10.1034/j.1600-0420.2000.078002146.x
Manges TD, Banaitis DA, Roth N, Yolton RL (1987) Changes in optometric findings during pregnancy. Am J Optom Physiol Opt 64:159–166. https://doi.org/10.1097/00006324-198703000-00001
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK). The authors received no funding for the research, authorship, and/or publication of this article from any government or private institution.
Author information
Authors and Affiliations
Contributions
ÇEP was responsible for designing the review protocol, writing the protocol and report, conducting the search, screening potentially eligible studies, extracting and analyzing data, interpreting results, writing the report, updating reference lists, and creating tables and figures. AÇY was responsible for designing the review protocol and screening potentially eligible studies. She contributed to writing the report, extracting and analyzing data, interpreting results, and creating tables.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
This study was approved by the Ethics Committee of Akdeniz University Faculty of Medicine (Approval Number KAEK-841). All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent to participate and publish Informed consent for publication of their clinical details and/or clinical images was obtained from all individual participants included in the study. A copy of the consent form is available for review by the editor of this journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Erkan Pota, Ç., Çetinkaya Yaprak, A. Evaluation of anterior segment parameters between pregnancy trimesters and postpartum with pentacam scheimflug ımaging: a prospective study. Int Ophthalmol 44, 268 (2024). https://doi.org/10.1007/s10792-024-03173-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10792-024-03173-y