Abstract
Purpose
To review all studies reporting the onset of white dot syndromes following COVID-19 vaccines.
Methods
Our protocol was registered prospectively on PROSPERO [registration number: CRD42023426012]. We searched five different databases including PubMed, Scopus, Web of Science, Google Scholar, and Science Direct up to May 2023. All the studies that reported the occurrence of white dot syndrome following COVID-19 vaccines were included. All statistical tests were conducted with a 95% confidence interval and a 5% error margin. A p value of less than 0.05 was considered statistically significant. The methodological quality of included studies was performed using the IHE Quality Appraisal Checklist for Case Series studies and JBI Critical Appraisal Checklist for Case Reports.
Results
Fifty studies involving seventy-one subjects were included. Multiple evanescent white dot syndrome (MEWDS) was the most common disease (n = 25, 35.2% %), followed by acute macular neuroretinopathy (AMN) (n = 22, 31.0%) and acute posterior multifocal placoid pigment epitheliopathy (APMPPE) (n = 4, 5.6%). They were mostly unilateral (n = 50, 70.4%). The presenting symptoms were blurred vision (n = 26, 36.6%), paracentral scotoma (n = 19, 26.8%), visual field disturbance, and photopsia (n = 7, 9.9%). The mean duration for follow-up was 10.15 ± 14.04 weeks. Nineteen subjects (29.69%) received steroids with improvement reported in 68.4%. Eleven subjects (17.19%) were managed by observation only with reported full recovery and improvement.
Conclusion
White dot syndromes are very rare entities. Our findings highlight a possible association between COVID-19 vaccines and the occurrence of white dot syndromes. However, larger studies with good quality should be implemented to confirm these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) pandemic has significant health consequences. Seven million fatalities have been reported from the 768 million confirmed cases of COVID-19 worldwide [1]. Nearly all the body's organs may be affected by the illness, which has the potential to cause respiratory issues ranging from those with no symptoms to those that are seriously uncomfortable or even fatal [2]. To control the COVID-19 pandemic, reliable and safe vaccines are crucial [3, 4]. As of the time of this writing, More than 13.4 billion doses of vaccines have been distributed [1] including messenger RNA vaccines (Pfizer-BioNTech and Moderna), inactivated vaccines (Sinopharm, Bharat Biotech, Sinovac), vector vaccines (Johnson & Johnson, AstraZeneca, Sputnik V), and protein subunit vaccinations (Novavax) [5]. Even though these vaccines were effective in halting the disease’s spread and lowering the incidence of severe forms of SARS-CoV-2, many potential adverse events were reported worldwide. [6, 7]
Until now, numerous unfavorable side effects, particularly those affecting the eyes, were reported [8]. For instance, some studies have reported that the Pfizer-BioNTech vaccine may cause about eye swelling, ocular hyperemia, conjunctivitis, blurred vision, uveitis, and visual impairment [6, 9]. Pfizer-BioNTech and AstraZeneca vaccines have been reported to result in ocular vascular events including vitreous hemorrhage, central/branch retinal vein occlusion, and ischemic optic neuropathy [10]. Mahendradas et al. [1] have reported anterior uveitis, intermediate uveitis, posterior uveitis, panuveitis, episcleritis, scleritis, sclerouveitis, sclerokeratouveitis, and keratouveitis following COVID-19 vaccines in their tertiary center.
White Dot Syndromes (WDS) are rare diseases of chorioretinopathy that have an annual incidence of 0.45 per 100,000 per year and typically affect young, healthy adults [11]. It has been reported that WDS can happen after vaccination for a number of diseases, including influenza, hepatitis B, polio, human papillomavirus (HPV), measles, mumps, rubella, and COVID-19 [12,13,14,15,16,17,18]. In fact, there growing evidence linking COVID-19 vaccinations and subtypes of WDS. For example, mRNA-1273 COVID-19 vaccine (Moderna) is associated with multiple evanescent white dot syndrome (MEWDS), and acute zonal occult outer retinopathy (AZOOR).[18, 19] In addition, acute macular neuroretinopathy (AMN), acute posterior multifocal placoid pigment epitheliopathy (APMPPE), and AZOOR have all been linked to the Pfizer-BioNTech vaccine administration. [17, 20,21,22,23] Furthermore, Medigen Vaccine Biologics Corporation (MVC) and Sinovac have been reported to cause MEWDS. [24, 25] Oxford-AstraZeneca, Sinopharm, and Johnson & Johnson also were reported to cause AMN [26,27,28,29].
The development of WDS following COVID-19 vaccination should therefore receive more attention. The importance of this recent possible association has been emphasized in several recent studies that have been published in this field but are solely based on case reports. As a result, we conducted a systematic review with a focus on the data regarding the vaccines (type, dose, duration), the patient's characteristics (sociodemographic and clinical), and the outcomes of the disease (origin, type, location, presentation, management, and outcomes) to summarize the current evidence on COVID-19 vaccine-associated WDS. This is the first systematic review that, to our knowledge, addresses WDS that develops following COVID-19 vaccination.
Materials and methods
Study protocol and database search
This research was carried out in accordance with the Preferred Reporting for Systematic Review and Meta-Analysis (PRISMA) recommendations [30, 31].The study adhered to the tenets of the Declaration of Helsinki and the necessity for institutional review board (IRB) approval was not required since it did not involve human subjects. In May 2023, our protocol was registered prospectively on PROSPERO [registration number: CRD42023426012].
Meanwhile, on May 25, 2023, we searched five electronic databases [PubMed, Scopus, Web of Science, ScienceDirect, and Google Scholar] to retrieve all studies that reported the onset of any type of WDS following COVID-19 vaccines within 8 weeks using the following keywords: (Pfizer-BioNTech OR BTN162b2 OR Sinopharm OR Sinovac OR Moderna OR AstraZeneca OR ChAdOx1 OR AZD1222 OR Janssen OR “Johnson & Johnson” OR Novavax OR CoronaVac OR Covaxin OR Convidecia OR Sputnik OR Zifivax OR Corbevax OR COVIran OR SCB-2019 OR vaccin* OR “COVID-19 Vaccines”[Mesh]) AND ((“white dot syndrome” OR “multiple evanescent white dot syndrome” OR “acute idiopathic blind spot enlargement syndrome” OR “punctate inner chorioretinop*” OR “acute macular neuroretinopath*” OR “acute posterior multifocal placoid pigment epitheliopathy” OR “diffuse subretinal fibrosis uveitis” OR “serpiginous choroid*” OR “birdshot chorioretinopathy*” OR “acute zonal occult outer retinopathy”))). Medical Subject Headings (MeSH) terms were also added whenever applicable to retrieve all relevant studies based on their indexed terms in included databases. In addition, only the first 200 records from Google Scholar were retrieved and screened as per the recent recommendations [30]. Noteworthy, an updated database search was carried out just before the analysis to include any newly published studies before the official synthesis of collected data which didn’t yield any new results.
Furthermore, after finishing the screening process, we conducted a manual search of references to identify any relevant studies that we could not identify through the original database search. This search was conducted by (1) searching similar articles of the finally included articles in our review through the “similar articles” option on PubMed, (2) searching the reference list of finally included articles in our review, and (3) searching through Google with the keywords used in the original database search.
Eligibility criteria
We included all original research papers that reported the onset of any type of WDSs following any type of COVID-19 vaccine within 8 weeks of vaccine administration following the PICO framework (Population: subjects with any type of WDS, Intervention: COVID-19 vaccines, Comparator: none, Outcome: presentation, management, and prognostic factors. We included all of the following study designs: randomized controlled clinical trials (RCT), retrospective and observational studies, case series, and case reports. Of note, studies were included regardless of the language of publication. Meanwhile, studies were excluded if they were (1) non-original research (i.e., reviews, commentaries, guidelines, editorials, correspondence, letters to editors, opinions etc.), (2) unavailable full-texts, (3) duplicated records or records with overlapping datasets, (4) studies reporting WDSs following SARS-CoV-2 infection (5) studies with irrelevant data (lack of primary outcome data) (6) studies reporting WDSs following COVID-19 vaccines within > 8 weeks.
Screening and study selection
Retrieved records from the database search were exported into EndNote software for duplicate removal before the beginning of the screening phase. Records were then imported into an Excel (Microsoft, USA) sheet for screening. The screening was divided into two steps: title and abstract screening followed by full-text screening. The full texts of eligible articles were then retrieved for screening before being finally included in the review. Both steps were carried out by three reviewers [AKH, ARH, AS]. Any differences between reviewers were solved through group discussions, and the senior authors [HAS, AGE] were consulted if reviewers could not reach an agreement.
Data extraction and assessment of methodological quality and risk of bias
The data extraction was performed by three reviewers [AKH, ARH, AS] through a data extraction sheet that was formatted through Excel (Microsoft, USA). This sheet consisted of five parts. The first part included the baseline characteristics of included studies [title, authors’ names, year of publication, country, and study design] and patients as well [sample size, age, and gender]. The second part included data on the reported WDS events (name, type, number, and laterality [right or left eye]), COVID-19 vaccine (type, number of doses, time from vaccine administration to symptoms onset, and SARS-CoV-2 infection status). The third part summarized the medical history of the reported cases with WDS events (i.e., systemic diseases, cardiovascular diseases, cerebrovascular diseases, immunological diseases, history of eye trauma, previous eye diseases, and previous ocular surgeries). The fourth part included a thorough assessment of the reported event in terms of presenting symptoms, diagnostic methods, examination findings, initial and final best-corrected visual acuity (BCVA), investigations (blood and eye investigations), management (either medical or surgical), the follow-up period, and management outcomes and associated complications if present. The fifth part included the quality assessment of the included studies. Methodological quality and risk of bias were assessed using the IHE Quality Appraisal Checklist for Case Series studies [32] and the JBI Critical Appraisal Checklist for Case Reports. [33]
Data synthesis
No modifications have been made to the pre-defined analysis plan in the study protocol. We performed qualitative analysis after organizing the acquired data. Qualitative analysis was done using the Statistical Package for Social Sciences (SPSS) version 27 (IBM SPSS Corp, SPSS Statistics ver. 27, USA). Descriptive analysis was used to display categorical variables as percentages and frequencies while presenting numerical variables as a mean and standard deviation. We tried to run time-to-event analysis for better understanding of relation of theWDS to the vaccines. The significance of the data was determined using a categorical Chi-square test. All statistical tests were conducted with a 95% confidence interval and a 5% error margin. A p-value of less than 0.05 was considered statistically significant. Visual acuity (VA) was commonly reported as an Early Treatment Diabetic Retinopathy Study letter scores. We standardized VA scores using the minimum angle of resolution (logMAR) chart scores, the score was converted to logMAR scores using Gregori et al. method. [34]
Results
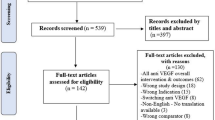
The search strategy retrieved 240 references and forty-five studies were included. (Fig. 1) Thirty-two were case reports (71.11%) [12, 18, 20, 24,25,26, 35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64] and 13 were case series (31.11%). [17, 65,66,67,68,69,70,71,72,73,74,75,76] Sixty-four patients were included. Their age average was 32.60 ± 13.76 (mean ± SD). Female patients were fifty-one (80.95%) and males were twelve (19.05%) and one case didn’t report the gender. The studies originated from twenty-two countries, Europe (n = 15, 33.33%), and USA (n = 10, 22.22%) were the most. (Table 1) The quality assessment and overall appraisal of the included articles is shown in Supplementary Table 1 and 2.
The reported COVID-19 vaccines-associated WDS were MEWDS (n = 33, 51.56%), AMN (n = 22 34.38%), APMPPE (n = 6, 9.38%), paracentral acute middle maculopathy (n = 1, 1.4%), multifocal choroiditis (n = 1, 1.56%), persistent placoid maculopathy (n = 1, 1.56%), and punctate inner choroidopathy (n = 1, 1.56%). Forty-five (70.31%) cases were unilateral while twenty 19 were bilateral (29.69%). Interestingly, all female cases presented with unilateral involvement except one case was bilateral. On the other hand, all males developed bilateral WDS. The mean duration of the WDS presentation was 18.88 ± 41.06 days while the median was 7 days. Twenty-six (40.63%) patients complained of blurred vision, followed by paracentral scotoma (n = 19, 15.63%), and photopsia in (n = 8, 12.50%) and visual field disturbance reported in six patients (n = 6, 9.38%). Regarding the treatment, nineteen patients (29.69%) received steroids. Among them, nine (47.37%) reported improvement from the baseline, four (21.05%) experienced complete resolution, one (5.26%) showed no improvement, and the outcome was not reported for five patients (26.32%). In addition, eleven patients (17.19%) were managed by observation only. Of them, five (45.45%) improved and the other six (54.55%) patients reported complete resolution. The management was not reported in 31 subjects, The average duration of treatment was 23.43 ± 23.46 days. The average duration of follow-up is 9.64 ± 13.43 weeks. (Table 2).
Regarding the patients’ previous ophthalmological history, six (9.38%) patients reported a previous history of ophthalmological issues. Myopia was present in five patients (83.33%), previous ocular surgeries/treatments reported in three patients (50%), and specific eye conditions (central serous chorioretinopathy with chronic serous pigment epithelial detachment and a previous episode of MEWDS) were found in two patients (33.33%). The mean baseline visual acuity (LogMAR) was 1.02 ± 0.43. (Table 1).
Optical coherence tomography (OCT) was conducted in forty-six fifty patients (71.88%). One patient only had normal OCT (2.17%) while the others reported mainly EZ disruption (n = 27, 58.70%), lesions and hyperreflective spots (n = 23, 50.00%), outer retinal layer abnormalities (n = 19, 41.30%), and subretinal fluid and detachments (n = 7, 15.22%). Moreover, fluorescein angiography (FA) was performed in twenty-three patients (35.94%) which were normal in three patients (13.04%) while the other patients reported hyperfluorescence with wreath-like pattern (n = 10, 43.48%), early hypofluorescence and late hyperfluorescence (n = 10, 43.48%), and late staining or leakage (n = 9, 39.13%). Fundus autofluorescence (FAF) was reported in thirteen patients (20.31%) which was normal in one patient (7.69%), and the other patients reported hyper autofluorescence (n = 9, 69.23%), while hypoautofluorescent (n = 10, 76.92%%). The mean number of doses that were administered to the patients was 1.47 ± 0.54. The mean duration from taking the vaccine till the onset of symptoms was 9.60 ± 10.66 days (mean ± SD). (Fig. 2) Complications of vaccination were reported in twelve patients (18.75%) including, but not limited to, flu-like symptoms (n = 4, 33.33%), and pain at the injection site (n = 4, 33.33%). (Table 1) Long-term ocular complications were observed in 13.4% of patients (n = 11). Most of them were persistent scotoma (n = 4, 36.7%). (Supplementary Table 3).
Discussion
Since the emergence of COVID-19 vaccines, many adverse events have been recognized globally. Of these adverse events, different types of WDS had been reported in the literature. Our results reviewed 82 cases received different COVID-19 vaccines. The most reported vaccine used was Pfizer (n = 23, 28%) in which showed a predominance of MEWDS (n = 15, 65.2%) and the rest of cases (n = 4, 17%) were diagnosed as AMPPE. AstraZeneca was seen in a total of 12 cases (14.6%), 10 cases of them diagnosed with AMN. Finally, Sinovac was administered in 16 cases (19%) of which 10 of them were associated with MEWDS and three of them were associated with AMN. Therefore, our data showed that Pfizer and AstraZeneca vaccine are associated mainly with MEWDS, and AMN, respectively. This could be explained by the fact that MEWDS is believed to be of an autoimmune nature given its autoimmune associations [11]. Pfizer–BioNTech vaccine produces an additional CD8 T-cell immune response triggering autoimmune reactions. [77] Nevertheless, this finding could also be explained by the dominance of the Pfizer-BioNTech vaccine over other COVID-19 vaccine types in the number of given doses worldwide [78]. Furthermore, AMN is hypothesized to be a systemic autoimmune disease that causes small-vessel occlusion due to micro-thrombi production, leading to ischemic retinopathy [79]. It has been reported that AstraZeneca vaccine provides protection against SARS-CoV-2 infection through immune-mediated mechanisms which are believed to cause thrombosis through an activation of platelets, immune cells, and hypercoagulability factors [10].
Our review included subjects with a mean age of 32.79 ± 14.81 years which is the typical age of WDS reported in the literature [82]. Because of the autoimmunity nature of WDS, it tends to occur more frequently in females [83]. Although it's unclear what's causing this trend, there is growing evidence that sex hormones affect the immune response, with estrogen enhancing and androgens suppressing it [84]. Moreover, it has been hypothesized that estrogen is crucial for the development and function of Th17 cells in addition to IL-17 generation [85].Our results coincide with this trend, showing that COVID-19 vaccine-associated WDS were more likely to occur in females than in males (77.5% vs. 21.1%).
The mean of the duration from taking the vaccine till the onset of symptoms was 10.06 ± 11.37 days (mean ± SD). In the literature, ocular adverse effects of COVID-19 vaccines, in general, happened in the first 10 days after administration of vaccines [86] .This temporal association could be explained by the fact that vaccine-related antibodies, that promote immune response including hypercoagulability, appear maximally within the first 5–10 days after vaccination, and disappear within 100 days [87]. In addition, 58.3% of WDS after AstraZeneca vaccine administration occurred within the first week, and all of them were AMN. Pfizer–BioNTech vaccine cases also were seen most frequently in the first week (46.1%) and second week (30.8%). On the other hand, Covishield and mRNA Spikvax-associated WDS were observed within 2 months of administration. (Supplementary Table 4).
Regarding OCT, VF and FAF findings in our study, the results were consistent with previously reported literature. The SD-OCT appearance of MEWDS is that of disruption mainly of the ellipsoid zone and interdigitation zone complex in the fovea and it is sometimes associated with reflective focal lesions that crossed the external limiting membrane line [80] and FA reveals early punctate hyperfluorescence in a wreath-like pattern and late staining, in areas corresponding to the white dots. This hyperfluorescence may be due to dilated retinal microcirculation in the middle or deep retinal capillary plexus [81].
Regarding VF complications, a total of nine patients (9.7%) reported complications of VF ch were mainly associated with AMN (n = 5, 62.5%) in the form of the typical VF defects (paracentral and temporal scotoma). Other 2 cases (n = 2, 25%) were associated with MEWDS in the form of enlarged blind spot and paracentral scotoma. Lastly, one case (n = 1, 12.5%) was associated with neuroretinitis in the form of superior scotoma and generalized field defect. Our results showed further analysis of the types of vaccine causing VF defect. Four cases of paracentral scotoma were found. Two of them were associated with AstraZeneca vaccine, one case was associated with MVC COVID-19 and Sinopharm vaccines, respectively. Enlarged blind spots and inferotemporal scotomas were seen in only one case, respectively, due to Spikevax vaccine. Interestingly, Pfizer–BioNTech vaccine has not been reported to cause VF defects.
Overall, the etiology of WDS is still uncertain and the emergence of cases under COVID-19 vaccines may shed some light on the exact pathogenesis of these syndromes. According to the WHO, as of the 21st of September 2023, 13.5 billion COVID-19 vaccine doses have been administered globally, and 27,338 are now administered each day [1]. Therefore, this possible association between COVID-19 vaccines and WDS might be a coincidence. In addition, the nature of case reports and series may introduce bias and limit the generalizability of our findings raising questionable associations. Further research is recommended to investigate these possible associations. The large number of studies included increases the discrepancy in reporting between different studies, hence a large group study could mitigate this effect and unify the reporting criteria for these syndromes.
Conclusion
Our review summarizes the occurrence of COVID-19 vaccination-associated WDS, which is more likely to occur among middle-aged females. Our findings indicate a possible association between COVID-19 vaccines and WDS, but this association is limited by the quality and number of available studies. The clinicians should be aware enough of this possible association and report them immediately upon the identification of similar cases for better implementation of the evidence. Further studies are needed for better determination of the incidence, risk factors, characteristics, and management of these syndromes.
Data and materials availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
WHO (2022) https://covid19.who.int/
Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC (2020) Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19) a review. JAMA J Am Med Assoc. https://doi.org/10.1001/jama.2020.12839
Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF (2021) Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol. https://doi.org/10.1038/s41577-021-00592-1
Krammer F (2020) SARS-CoV-2 vaccines in development. Nature. https://doi.org/10.1038/s41586-020-2798-3
Hadj Hassine I (2022) Covid-19 vaccines and variants of concern: a review. Rev Med Virol. https://doi.org/10.1002/rmv.2313
Nyankerh CNA, Boateng AK, Appah M (2022) Ocular complications after COVID-19 vaccination, vaccine adverse event reporting system. Vaccines (Basel) 10(6):941. https://doi.org/10.3390/vaccines10060941
Sen M, Honavar S (2021) After the storm: ophthalmic manifestations of COVID-19 vaccines. Indian J Ophthalmol 69(12):3398. https://doi.org/10.4103/ijo.IJO_2824_21
Ichhpujani P, Parmar UPS, Duggal S, Kumar S (2022) COVID-19 vaccine-associated ocular adverse effects: an overview. Vaccines (Basel) 10(11):1879. https://doi.org/10.3390/vaccines10111879
Cherif YYS et al (2023) The characteristics of COVID-19 vaccine-associated uveitis: a summative systematic review. Vaccines. https://doi.org/10.3390/vaccines11010069
Abu Serhan H et al (2022) Ocular vascular events following COVID-19 vaccines: a systematic review. Vaccines. https://doi.org/10.3390/vaccines10122143
Abu-Yaghi NE, Hartono SP, Hodge DO, Pulido JS, Bakri SJ (2011) White dot syndromes: a 20-year study of incidence, clinical features, and outcomes. Ocul Immunol Inflamm 19(6):426–430. https://doi.org/10.3109/09273948.2011.624287
Jakirlic N, Harris T (2022) Case report: acute posterior multifocal placoid pigment epitheliopathy after SARS-CoV-2 vaccination. Optom Vis Sci. https://doi.org/10.1097/OPX.0000000000001900
Kraemer LS, Montgomery JR, Baker KM, Colyer MH (2022) Acute posterior multifocal placoid pigment epitheliopathy after immunization with multiple vaccines. Retin Cases Brief Rep. https://doi.org/10.1097/ICB.0000000000000959
Wong M, Campos-Baniak MG, Colleaux K (2019) Acute idiopathic blind spot enlargement syndrome following measles, mumps and rubella vaccination. Can J Ophthalmol 54(4):e199–e203. https://doi.org/10.1016/j.jcjo.2018.09.005
Abou-Samra A, Tarabishy AB (2019) Multiple evanescent white dot syndrome following intradermal influenza vaccination. Ocular Immunol Inflam. https://doi.org/10.1080/09273948.2017.1423334
Ogino K, Kishi S, Yoshimura N (2014) Multiple evanescent white dot syndrome after human papillomavirus vaccination. Case Rep Ophthalmol. https://doi.org/10.1159/000358870
Bouhout S, Hébert M, Vadboncoeur J, Aubin MJ (2023) Multiple evanescent white dot syndrome following COVID-19 vaccines. Can J Ophthalmol. https://doi.org/10.1016/j.jcjo.2022.10.002
Soifer M, Nguyen NV, Leite R, Fernandes J, Kodati S (2022) Recurrent Multiple Evanescent white dot syndrome (MEWDS) following first dose and booster of the mRNA-1273 COVID-19 vaccine: case report and review of literature. Vaccines (Basel). https://doi.org/10.3390/vaccines10111776
Yasaka Y et al (2023) A multicenter study of ocular inflammation after COVID-19 vaccination. Jpn J Ophthalmol. https://doi.org/10.1007/s10384-022-00962-9
Atas F, Kaya M, Saatci AO (2023) Acute multifocal placoid pigment epitheliopathy-like presentation following the first dose of BNT162B2 COVID-19 vaccination. Ocular Immunol Inflamm. https://doi.org/10.1080/09273948.2021.1995763
Nagaoka K, Makino S (2023) Acute multifocal placoid pigment epitheliopathy following administration of the first dose of the BNT162B2 COVID-19 vaccine. QJM. https://doi.org/10.1093/qjmed/hcac253
Maleki A, Look-Why S, Manhapra A, Foster CS (2021) COVID-19 recombinant mRNA vaccines and serious ocular inflammatory side effects: Real or coincidence? J Ophthalmic Vis Res. https://doi.org/10.18502/jovr.v16i3.9443
Menteş J, Nalçacı S, Değirmenci C (2023) A case of concurrent acute macular neuroretinopathy and paracentral acute middle maculopathy following Pfizer-BioNTech COVID-19 vaccination. Turk J Ophthalmol. https://doi.org/10.4274/tjo.galenos.2023.65118
Lin KS, Hsieh MH (2022) Multiple evanescent white dot syndrome following medigen vaccine biologics corporation COVID-19 vaccination. Ocular Immunol Inflamm. https://doi.org/10.1080/09273948.2022.2062388
Tomishige KS, Novais EA, Luciana LP, H. M. do Nascimento, and R. Belfort, (2022) Multiple evanescent white dot syndrome (MEWDS) following inactivated COVID-19 vaccination (Sinovac-CoronaVac). Arq Bras Oftalmol. https://doi.org/10.5935/0004-2749.20220070
Baharani A, Reddy RR (2023) Multiple evanescent white dot syndrome following adenovirus vector-based COVID-19 vaccine (Covishield). Ocul Immunol Inflamm. https://doi.org/10.1080/09273948.2023.2192271
Vinzamuri S, Pradeep TG, Kotian R (2021) Bilateral paracentral acute middle maculopathy and acute macular neuroretinopathy following COVID-19 vaccination. Indian J Ophthalmol. https://doi.org/10.4103/ijo.IJO_1333_21
Patel SN, Yonekawa Y (2022) Acute macular neuroretinopathy after SARS-COV-2 vaccination. Retin Cases Brief Rep. https://doi.org/10.1097/ICB.0000000000001195
Fekri S, Khorshidifar M, Dehghani MS, Nouri H, Abtahi SH (2023) Acute macular neuroretinopathy and COVID-19 vaccination: case report and literature review. J Francais d’Ophtalmologie. https://doi.org/10.1016/j.jfo.2022.09.008
Muka T et al (2020) A 24-step guide on how to design, conduct, and successfully publish a systematic review and meta-analysis in medical research. Eur J Epidemiol. https://doi.org/10.1007/s10654-019-00576-5
Page MJ et al (2020) The PRIMSA statement: an updated guideline for reporting systematic reviews. The BMJ. https://doi.org/10.1136/BMJ.N71
“(IHE), I.o.H.E., Quality appraisal of case series studies checklist
Chapter 7: Systematic reviews of etiology and risk. JBI Manual for Evidence Synthesis, 2020. https://doi.org/10.46658/jbimes-20-08
Gregori NZ, Feuer W, Rosenfeld PJ (2010) Novel method for analyzing snellen visual acuity measurements. Retina. https://doi.org/10.1097/IAE.0b013e3181d87e04
Alhabshan R, Scales D (2022) Multiple evanescent white dot syndrome developing three days following administration of mRNA-1273 booster vaccine: case report. Case Rep Ophthalmol. https://doi.org/10.1159/000525687
Beketova TR, Snyder K, Jiang A, Josephberg RG (2023) Acute posterior multifocal placoid pigment epitheliopathy with associated papillitis. Cureus. https://doi.org/10.7759/cureus.35499
Bellur S, Zeleny A, Patronas M, Jiramongkolchai K, Kodati S (2022) Bilateral acute macular neuroretinopathy after COVID-19 vaccination and infection. Ocul Immunol Inflamm. https://doi.org/10.1080/09273948.2022.2093753
Bøhler AD, Strøm ME, Sandvig KU, Moe MC, Jørstad ØK (2021) “Acute macular neuroretinopathy following COVID-19 vaccination. Eye 36(3):644–645. https://doi.org/10.1038/s41433-021-01610-1
Book BAJ, Schmidt B, Foerster AMH (2021) Bilateral acute macular neuroretinopathy after vaccination against SARS-CoV-2. JAMA Ophthalmol. https://doi.org/10.1001/jamaophthalmol.2021.2471
Chean CS, Ali E, Kulkarni P, Kapoor B, Kumar P (2023) Bilateral persistent placoid maculopathy following COVID-19 vaccines: real or coincidence? Ocul Immunol Inflamm. https://doi.org/10.1080/09273948.2023.2170889
Drüke D, Pleyer U, Hoerauf H, Feltgen N, Bemme S (2021) Acute macular neuroretinopathy (AMN) following COVID-19 vaccination. Am J Ophthalmol Case Rep. https://doi.org/10.1016/j.ajoc.2021.101207
Fekri S, Khorshidifar M, Dehghani MS, Nouri H, Abtahi SH (2023) Acute macular neuroretinopathy and COVID-19 vaccination: case report and literature review. J Fr Ophtalmol 46(1):72–82. https://doi.org/10.1016/J.JFO.2022.09.008
Gabrielle PH et al (2022) Bilateral acute macular neuroretinopathy in a young woman after the first dose of Oxford–AstraZeneca COVID-19 vaccine. Am J Ophthalmol Case Rep. https://doi.org/10.1016/j.ajoc.2022.101281
Goyal M, Murthy SI, Annum S (2021) Bilateral multifocal choroiditis following COVID-19 vaccination. Ocular Immunol Inflamm. https://doi.org/10.1080/09273948.2021.1957123
Hébert M, Couture S, Schmit I (2019) Bilateral panuveitis with occlusive vasculitis following coronavirus disease vaccination. Ocul Immunol Inflamm 31(3):2023. https://doi.org/10.1080/09273948.2022.2042325
Inagawa S et al (2022) Multiple evanescent white dot syndrome following vaccination for COVID-19 A case report. Medicine (United States). https://doi.org/10.1097/MD.0000000000028582
Lee C et al (2022) Neuroretinitis after the second injection of a SARS-CoV-2-vaccine: A case report. Am J Ophthalmol Case Rep. https://doi.org/10.1016/j.ajoc.2022.101592
McElhinney K, McGrath R, Ahern E, O’Connell E (2022) Bilateral acute posterior multifocal placoid pigment epitheliopathy (APMPPE) following SARS-CoV-2 mRNA vaccine. BMJ Case Reports. https://doi.org/10.1136/bcr-2022-250346
Michel T et al (2022) Acute macular neuroretinopathy after COVID-19 vaccine. J Fr Ophtalmol 45(7):e299–e302. https://doi.org/10.1016/J.JFO.2022.01.022
Nair GS, Khan IA, Rizvi SWA, Shahid S (2023) A case of neuroretinitis following inactivated virion COVID-19 vaccination. Ocul Immunol Inflamm. https://doi.org/10.1080/09273948.2023.2173244
Ninet L, Comet A, Denis D, David T (2022) Multiple evanescent white dot syndrome following BioNTech SARS-CoV2 mRNA vaccination. J Francais d’Ophtalmologie. https://doi.org/10.1016/j.jfo.2022.03.002
Ogino Y et al (2023) A case of APMPPE-like panuveitis presenting with extensive outer retinal layer impairment following COVID-19 vaccination. BMC Ophthalmol. https://doi.org/10.1186/s12886-023-02978-2
Patel SN, Yonekawa Y (2022) acute macular neuroretinopathy after SARS-COV-2 vaccination. Retin Cases Brief Rep 16(1):5–8. https://doi.org/10.1097/ICB.0000000000001195
Pichi F, Aljneibi S, Neri P, Hay S, Dackiw C, Ghazi NG (2021) Association of ocular adverse events with inactivated COVID-19 vaccination in patients in Abu Dhabi. JAMA Ophthalmol. https://doi.org/10.1001/jamaophthalmol.2021.3477
Abrishami M, Hosseini S, Shoeibi N, Heidarzadeh H (2019) Unilateral acute central serous chorioretinopathy with inactivated coronavirus disease vaccination: a case report and review of literature. J Curr Ophthalmol 34(3):2022. https://doi.org/10.4103/joco.joco_41_22
Rennie AT, DeWeerd AJ, Martinez MG, Kay CN (2022) Acute macular neuroretinopathy following COVID-19 mRNA vaccination. Cureus. https://doi.org/10.7759/cureus.27502
Sanjay S et al (2022) Bilateral sequential acute macular neuroretinopathy in an asian indian female with β thalassemia trait following (corona virus disease) COVID-19 vaccination and probable recent COVID infection - multimodal imaging study. Ocular Immunol Inflamm. https://doi.org/10.1080/09273948.2022.2026978
Seong HJ, Lee CS (2022) Multiple evanescent white dot syndrome with submacular fluid in dome-shaped macula following COVID-19 vaccination: a case report. Korean J Ophthalmol. https://doi.org/10.3341/kjo.2022.0077
Valenzuela DA, Groth S, Taubenslag KJ, Gangaputra S (2021) Acute macular neuroretinopathy following Pfizer-BioNTech COVID-19 vaccination. Am J Ophthalmol Case Rep. https://doi.org/10.1016/J.AJOC.2021.101200
Vinzamuri S, Pradeep TG, Kotian R (2021) Bilateral paracentral acute middle maculopathy and acute macular neuroretinopathy following COVID-19 vaccination. Indian J Ophthalmol 69(10):2862–2864. https://doi.org/10.4103/IJO.IJO_1333_21
Wiley ZC, Pakravan M, Charoenkijkajorn C, Kavoussi SC, Lee AG (2022) Uveomeningeal syndrome presenting with bilateral optic disc edema and multiple evanescent white dots syndrome (MEWDS). Am J Ophthalmol Case Rep. https://doi.org/10.1016/j.ajoc.2022.101538
Xu Y, Shen W (2021) Presumed Recurrent MEWDS following Covid-19 vaccination. Ocular Immunol Inflamm. https://doi.org/10.1080/09273948.2021.1985524
Yasuda E et al (2022) Multiple evanescent white dot syndrome following BNT162b2 mRNA COVID-19 vaccination. Am J Ophthalmol Case Rep. https://doi.org/10.1016/j.ajoc.2022.101532
Zaheer N, Renju MP, Chavan R (2022) ACUTE macular neuroretinopathy after Covid-19 vaccination. Retin Cases Brief Rep 16(1):9–11. https://doi.org/10.1097/ICB.0000000000001196
Arora A et al (2023) Recurrence of tubercular choroiditis following anti-SARS-CoV-2 vaccination. Eur J Ophthalmol. https://doi.org/10.1177/11206721221088439
Bolletta E et al (2021) Uveitis and other ocular complications following covid-19 vaccination. J Clin Med. https://doi.org/10.3390/jcm10245960
Franchi A et al (2022) Two cases of acute macular neuroretinopathy associated with the adenovirus-based COVID-19 vaccine vaxzevria (Astrazeneca). Ocular Immunol Inflamm. https://doi.org/10.1080/09273948.2022.2027463
Gargouri MA et al (2022) Multiple evanescent white dot syndrome following COVID-19 mRNA vaccination. Ocul Immunol Inflamm. https://doi.org/10.1080/09273948.2022.2127782
Girbardt C et al (2021) Retinal vascular events after mrna and adenoviral-vectored covid-19 vaccines—a case series. Vaccines (Basel). https://doi.org/10.3390/vaccines9111349
Ishibashi K, Yatsuka H, Haruta M, Kimoto K, Yoshida S, Kubota T (2022) Branch retinal artery occlusions, paracentral acute middle maculopathy and acute macular neuroretinopathy after COVID-19 vaccinations. Clin Ophthalmol. https://doi.org/10.2147/OPTH.S357359
Li S et al (2023) Intraocular inflammation following COVID-19 vaccination: the clinical presentations. Int Ophthalmol 43(8):2971–2981. https://doi.org/10.1007/S10792-023-02684-4/TABLES/2
Li Z et al (2022) Ocular adverse events after inactivated COVID-19 vaccination. Vaccines (Basel). https://doi.org/10.3390/vaccines10060918
Mambretti M, Huemer J, Torregrossa G, Ullrich M, Findl O, Casalino G (2019) acute macular neuroretinopathy following coronavirus disease vaccination. Ocul Immunol Inflamm 29(4):2021. https://doi.org/10.1080/09273948.2021.1946567
Rabinovitch T et al (2021) Uveitis following the BNT162b2 mRNA vaccination against SARS-CoV-2 infection: a possible association. Retina. https://doi.org/10.1097/IAE.0000000000003277
Smith E, Tran T, Gillies A, Yeung S, Ma PE (2022) Multiple evanescent white dot syndrome following COVID-19 mRNA vaccination in two patients. Ocular Immunol Inflamm. https://doi.org/10.1080/09273948.2022.2032198
Wang LU et al (2022) Ocular inflammatory manifestations following COVID-19 vaccinations in Taiwan: a case series. Taiwan J Ophthalmol. https://doi.org/10.4103/2211-5056.353129
Vogel AB et al (2021) BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature. https://doi.org/10.1038/s41586-021-03275-y
Our World in Data. COVID-19 vaccine doses administered by manufacturer, European Union. Available online: https://ourworldindata.org/grapher/covid-vaccine-doses-by-manufacturer (accessed on 14 September 2023)
Abu El-Asrar AM, Herbort CP, Tabbara KF (2009) Differential diagnosis of retinal vasculitis. Middle East Afr J Ophthalmol, 16(4)
Papasavvas I, Mantovani A, Tugal-Tutkun I, Herbort CP (2021) Multiple evanescent white dot syndrome (MEWDS): update on practical appraisal, diagnosis and clinicopathology; a review and an alternative comprehensive perspective. J Ophthal Inflamm Infect. https://doi.org/10.1186/s12348-021-00279-7
Tavallali A, Yannuzzi L (2017) MEWDS, common cold of the retina. J Ophthalm Vis Res. https://doi.org/10.4103/jovr.jovr_241_16
Quillen DA et al (2004) The white dot syndromes. Am J Ophthalmol 137(3):538–550. https://doi.org/10.1016/j.ajo.2004.01.053
Yeung IYL, Popp NA, Chan CC (2015) The role of sex in uveitis and ocular inflammation. Int Ophthalmol Clin. https://doi.org/10.1097/IIO.0000000000000072
Choudhary MM, Hajj-Ali RA, Lowder CY (2014) Gender and ocular manifestations of connective tissue diseases and systemic vasculitides. J Ophthalmol. https://doi.org/10.1155/2014/403042
Singh RP et al (2014) Th17 cells in inflammation and autoimmunity. Autoimmunity Rev. https://doi.org/10.1016/j.autrev.2014.08.019
Haseeb AA, Solyman O, Abushanab MM, Obaia ASA, Elhusseiny AM (2022) Ocular complications following vaccination for COVID-19: a one-year retrospective. Vaccines. https://doi.org/10.3390/vaccines10020342
Jalink MB, Bronkhorst IHG (2022) A sudden rise of patients with acute macular neuroretinopathy during the COVID-19 pandemic. Case Rep Ophthalmol. https://doi.org/10.1159/000522080
Acknowledgements
Open Access funding is provided by the Qatar National Library.
Funding
Open Access funding provided by the Qatar National Library. NA.
Author information
Authors and Affiliations
Contributions
Conceptualization: HAS, AGE; Data curation: HAS, HAS, AKH; Formal analysis: JFA, AS; Funding acquisition: HAS; Investigation: AS, ARH; Methodology: HAS, AGE, AA; Project administration: HAS, NA, AGE; Resources: HAS, AKH; Software; ARH, JFA; Supervision: AGE, HAS; Validation: HAS, HAS, AKH; Visualization: HAS; Roles/Writing—original draft: HAS, AGE, JFA; Writing—review & editing: HAS, AGE, AKH. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abu Serhan, H., Abu Suilik, H., Hassan, A.K. et al. The characteristics of white dot syndromes following COVID-19 Vaccines: a systematic review. Int Ophthalmol 44, 189 (2024). https://doi.org/10.1007/s10792-024-03119-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10792-024-03119-4