Abstract
Purpose
We aim to contribute to the literature in terms of treatment safety with our real world data by examining the anterior segment complications and follow-up results of patients who underwent dexamethasone implants in our clinic.
Methods
The records of patients treated with at least one intravitreal dexamethasone implant for various retinal diseases: diabetic macular edema (265 eyes), central retinal vein occlusion (45 eyes), retinal vein branch occlusion (91 eyes), postoperative cystoid macular edema (18 eyes), non-infectious uveitis (37 eyes) and other (14 eyes) between July 2013 and April 2020 were reviewed.
Results
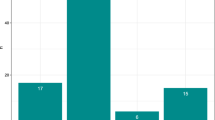
After 925 injections were applied to 470 eyes of a total of 383 patients, the eyes were controlled during a mean follow-up of 24 months. No complications were detected in 328 eyes. Intraocular pressure (IOP) above 25 mmHg was detected in 97 eyes (20.6%) that had no previous history of ocular hypertension. Of these 97 eyes, 71 (73.1%) eyes with increased IOP were treated with topical monotherapy, 26 (26.8%) eyes were treated with topical combined therapy and 1 (1.03%) patient had glaucoma surgery. Cataracts requiring surgical intervention developed in 55 (%21.73) of 253 phakic eyes. Three patients have anterior chamber dislocation of dexamethasone, 1 patient was hospitalized with sterile endophthalmitis on the 7th day after the injection, and pars plana vitrectomy was performed.
Conclusion
This study is the first long-term follow-up study in our country evaluating the safety of dexamethasone implant injections in various retinal diseases and presenting the first real world data. Cataract progression and increased IOP were found to be the most common side effects. We observed that the patient’s diagnosis did not cause a statistically significant change in the observation of side effects. As a result of our findings, close follow-up of IOP after the injection of dexamethasone implants would be appropriate.
Similar content being viewed by others
References
Pelzek C, Lim JI (2002) Diabetic macular edema: review and update. Ophthalmol Clin North Am 15:555–563
Scholl S, Kirchhof J, Augustin AJ (2010) Pathophysiology of macular edema. Ophthalmologica 224(Suppl 1):8–15
Bressler SB, Qin H, Beck RW et al (2012) Diabetic retinopathy clinical research network. Factors associated with changes in visual acuity and central subfield thickness at 1 year after treatment for diabetic macular edema with ranibizumab. ArchOphthalmol 130:1153–1161
Brown DM, Campochiaro PA, Singh RP et al (2010) Ranibizumab for macular edema following central retinal vein occlusion: 6-month primary end point results of a phase III study. Ophthalmology 117(6):1124–1133. https://doi.org/10.1016/j.ophtha.2010.02.022
Hykin P, Prevost AT, Vasconcelos JC et al (2019) Clinical effectiveness of intravitreal therapy with ranibizumab versus aflibercept versus bevacizumab for macular edema secondary to central retinal vein occlusion: a randomized clinical trial. JAMA Ophthalmol 137(11):1256–1264. https://doi.org/10.1001/jamaophthalmol.2019.3305
Mishra SK, Gupta A, Patyal S et al (2018) Intravitreal dexamethasone implant versus triamcinolone acetonide for macular edema of central retinal vein occlusion: quantifying efficacy and safety. Int J Retin Vitr 4:13. https://doi.org/10.1186/s40942-018-0114-2
Boyer DS, Yoon YH, Belfort RJ et al (2014) Ozurdex MEAD studygroup. Three-year, randomized, sham-controlled trial dexamethasone intravitreal implants in patients with diabetic macular edema. Ophthalmology 121:1904–1914
Blanc J, Kodjikian L, Bron A, Creuzot-Garcher C (2018) Response to safety and long-term efficacy of repeated dexamethasone intravitreal implant for the treatment of cystoid macular edema secondary to retinal vein occlusion with and without a switch to anti-VEGF agents: a 3-year experience. Graefe’s Arch Clin Exp Ophthalmol 256(11):2271–2271
Meyer LM, Schönfeld C-L (2013) Secondary glaucoma after intravitreal dexamethasone 0.7 mg implant in patients with retinal vein occlusion: a 1-year follow-up. J OculPharmacolTher 29:560–565
Schmitz K, Maier M, Clemens CR et al (2014) Reliability and safety of intravitreal Ozurdex injections. The ZERO study. Ophthalmologe 111:44–52
Malclès A, Dot C, Voirin N et al (2017) Safety of intravitreal dexamethasone implant (Ozurdex): the SAFODEX study. Incidence and risk factors of ocular hypertension. Retina 37:1352–1359
Russo A, Avitabile T, Uva M et al (2012) Radiation macular edema after Ru-106 plaque brachytherapy for choroidal melanoma resolved by an intravitreal dexamethasone 0.7-mg implant. Case RepOphthalmol 3:71–76
Meyer LM, Schönfeld C-L (2011) Cystoid macular edema after complicated cataract surgery resolved by an intravitreal dexamethasone 0.7-mg implant. Case RepOphthalmol 2:319–322
Klamann A, Böttcher K, Ackermann P et al (2016) Intravitreal dexamethasone implant for the treatment of postoperative macular edema. Ophthalmologica 236:181–185
Lei S, Lam W-C (2015) Efficacy and safety of dexamethasone intravitreal implant for refractory macular edema in children. Can J Ophthalmol 50:236–241
MelloFilho P, Andrade G, Maia A et al (2019) Effectiveness and safety of intravitreal dexamethasone implant (Ozurdex) in patients with diabetic macular edema: a real World experience. Ophthalmologica 241:9–16
He Y, Ren XJ, Hu BJ, Lam WCLX (2018) A meta-analysis of the effect of a dexamethasone intravitreal implant versus intravitreal anti-vascular endothelial growth factor treatment for diabetic maculare dema. BMC Ophthalmol 18(1):121
Haller JA, Bandello F, Belfort R et al (2011) Dexamethasone intravitreal implant in patients with macular edema related to branch or central retinal veinocclusion 12-month study results. Ophthalmology 118:2453–2460
Li X, Wang N, Liang X (2018) Safetyandefficacy of dexamethasone intravitreal implant for treatment of macular edema secondary to retinal vein occlusion in Chinese patients: randomized, sham-controlled, multicenter study. Graefes Arch Clin Exp Ophthalmol 256:59–69
Eter N, Mohr A, Wachtlin J (2017) Dexamethasone intravitreal implant in retinal vein occlusion:real-life data from a prospective, multicenter clinical trial. Graefes Arch Clin Exp Ophthalmol 255:77–87
Nagpal M, Mehrotra N, Juneja R et al (2018) Dexamethasone implant (0.7 mg) in Indianpatients with macular edema: real-life scenario. Taiwan J Ophthalmol 8:141–148
Zarranz-Ventura J, Carreño E, Johnston RL et al (2014) Multicenter study of intravitreal dexamethasone implant in noninfectious uveitis: indications, outcomes, andreinjectionfrequency. Am J Ophthalmol 158:1136–1145
Mayer WJ, Wolf A, Kernt M et al (2013) Twelve-month experience with Ozurdex for the treatment of macular edema associated with retinal vein occlusion. Eye 27:816–822
Srinivasan Rekha, Sharma Unnati, George Ronnie, Raman Rajiv, Sharma Tarun, Sankara Nethralaya Vitreoretinal Study Group (2019) Intraocular pressure changes after dexamethasone implant in patients with glaucoma and steroid responders. Retina 39(1):157–162. https://doi.org/10.1097/iae.0000000000001924
Gillies MC, Lim LL, Campain A et al (2014) randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macularedema: the BEVORDEX study. Ophthalmology. 121(12):2473–2481. https://doi.org/10.1016/j.ophtha.2014.07.002
Jirarattanasopa P, Jiranoppasakdawong S, Ratanasukon M, Bhurayanontachai P, Dangboon W (2022) Results of intravitreal dexamethasone implant (Ozurdex®) for retinal vascular diseases with macular edema: an observational study of real-life situations. Medicine (Baltimore) 101(27):e29807. https://doi.org/10.1097/MD.0000000000029807
Scott IU, Ip MS, VanVeldhuisen PC et al (2009) SCORE study research group. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular edema secondary to branch retinal vein occlusion: the standard care versus corticosteroid for retinal vein occlusion (SCORE) study report 6. Arch Ophthalmol 127:1115–1122
Funding
No financial or funding support was received for this research.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by YA, İB and ÇEP. The first draft of the manuscript was written by İB and ÇEP all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no confict of interest.
Ethical approval
This study was approved by the Ethics Committee of the Akdeniz University Medical Faculty. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent to participate and publish Informed consent for publication of their clinical details and/or clinical images was obtained from all individual participants included in the study. A copy of the consent form is available for review by the Editor of this journal.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ayaz, Y., Erkan Pota, Ç., Başol, İ. et al. Anterior segment complications after dexamethasone implantations:real world data. Int Ophthalmol 43, 4279–4287 (2023). https://doi.org/10.1007/s10792-023-02838-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-023-02838-4