Abstract
Purpose
To measure the lesion size reduction in eyes with active toxoplasma retinochoroiditis during the disease course with swept-source optical coherence tomography angiography (SS-OCTA).
Methods
We retrospectively analysed the chorioretinal lesion size in a group of 14 eyes with a single active toxoplasma retinochoroiditis lesion. SS-OCTA was performed at the baseline and follow-up in all eyes. The 6 × 6 mm choriocapillaris slab images were evaluated with image analysis (MATLAB). The number of black and white pixels in a 1500-µm-diameter circle centred on each active lesion was counted at the time of baseline examination and at the first follow-up visit when the chorioretinal scar formation was noticed.
Results
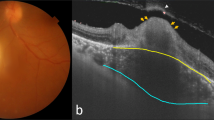
Fourteen eyes with a single active toxoplasmosis retinochoroiditis lesion were included. Ten patients were female and three were male. The mean age was 29.1 ± 14.9 years. Active lesions were at the macula in five eyes, at the periphery in six eyes and juxtapapillary in three eyes. At the initial examination, the lesion area was observed as an area with a decreased flow signal on SS-OCTA. There was the perilesional capillary disruption in superficial and deep capillary plexi together with a diffuse capillary network attenuation and non-detectable flow signal zones in the choriocapillaris slabs. In addition to sulfamethoxazole-trimethoprim and azithromycin combination, oral corticosteroids were only co-administered in five (35%) eyes with macular involvement. The chorioretinal scar formation was observed in 4 to 16 weeks. At the time of inactivity, the original lesion was diminished in size when compared to its baseline in all study eyes (p = 0.001) with a mean black pixel reduction percentage of 21.8%. The reduction was 15.4% in eyes with macular lesion, 31.6% with peripheral lesions and 18.1% with juxtapapillary lesions (p = 0.001, p = 0.032, p = 0.028, p = 0.043, respectively). Visual acuity was correlated with black pixel reduction percentage in eyes with macular lesion (r = 0.56, p < 0.001).
Conclusion
Healing of the active toxoplasma retinochoroiditis lesion size could be monitored with an OCTA-based image analysis technique. Interestingly, the reduction in the lesion size was lesser in the macular lesions than the peripheral and juxtapapillary lesions following the treatment and this might contribute to the poorer visual outcomes observed in eyes with macular lesions.


Similar content being viewed by others
Data availability
All data obtained during the study process were used in the study.
References
Yalcindag FN, Ozdal PC, Ozyazgan Y et al (2018) Demographic and clinical characteristics of Uveitis in Turkey: the first national registry report. Ocul Immunol Inflamm 26:17–26. https://doi.org/10.1080/09273948.2016.1196714
Lujan BJ (2014) Spectral domain optical coherence tomography imaging of punctate outer retinal toxoplasmosis. Saudi J Ophthalmol 28:152–156. https://doi.org/10.1016/j.sjopt.2014.03.010
Mathis T, Delaunay B, Favard C, Denis P, Kodjikian L (2020) Hyperautofluorescent spots in acute ocular toxoplasmosis: a new indicator of outer retinal inflammation. Retina 40:2396–2402. https://doi.org/10.1097/IAE.0000000000002759
Yoshizumi MO (1976) Experimental toxoplasma retinitis: a light and electron microscopical study. Arch Pathol Lab Med 100:487–490
Vainisi SJ, Campbell LH (1969) Ocular toxoplasmosis in cats. J Am Vet Med Assoc 154:141–152
Dingerkus VLS, Munk MR, Brinkmann MP et al (2019) Optical coherence tomography angiography (OCTA) as a new diagnostic tool in uveitis. J Ophthalmic Inflamm Infect 9:10. https://doi.org/10.1186/s12348-019-0176-9
Yeo JH, Chung H, Kim JT (2019) Swept-source optical coherence tomography angiography according to the type of choroidal neovascularization. J Clin Med 8:1272. https://doi.org/10.3390/jcm8091272
Jabs DA, Nussenblatt RB, Rosenbaum JT, Standardization of Uveitis Nomenclature Working G (2005) Standardization of uveitis nomenclature for reporting clinical data. Results of the first international workshop. Am J Ophthalmol 140:509–516. https://doi.org/10.1016/j.ajo.2005.03.057
Holland GOCG, Belfort R, Remington JS (1996) Toxoplasmosis. In: Pepose JS, Holland GN, Wilhelmus KR (eds) Ocular infection and immunity. Mosby-Year Book Inc, Missouri, St Louis, pp 1183–1223
Otsu N (1979) A threshold selection method from gray-level histograms. IEEE Trans Syst Man Cybern 9:62–66. https://doi.org/10.1109/tsmc.1979.4310076
Cohen LM, Goldstein DA, Fawzi AA (2016) Structure-function relationships in uveitic cystoid macular edema: using en face optical coherence tomography to predict vision. Ocul Immunol Inflamm 24:274–281. https://doi.org/10.3109/09273948.2015.1056535
Karti O, Saatci AO (2019) Optical coherence tomography angiography in eyes with non-infectious posterior uveitis; some practical aspects. Med Hypothesis Discov Innov Ophthalmol 8:312–322
de Oliveira Dias JR, Campelo C, Novais EA et al (2020) New findings useful for clinical practice using swept-source optical coherence tomography angiography in the follow-up of active ocular toxoplasmosis. Int J Retina Vitreous 6:30. https://doi.org/10.1186/s40942-020-00231-2
Park JH, Lee SY, Lee EK (2019) Morphological characteristics of ocular toxoplasmosis and its regression pattern on swept-source optical coherence tomography angiography: a case report. BMC Ophthalmol 19:199. https://doi.org/10.1186/s12886-019-1209-8
Leong BCS, Gal-Or O, Freund KB (2019) Optical coherence tomography angiography of retinal-choroidal anastomosis in toxoplasmosis chorioretinitis. JAMA Ophthalmol 137:e184091. https://doi.org/10.1001/jamaophthalmol.2018.4091
Spaide RF (2015) Volume rendering of optical coherence tomography angiography reveals extensive retinal vascular contributions to neovascularization in ocular toxoplasmosis. Retina 35:2421–2422. https://doi.org/10.1097/IAE.0000000000000721
Azar G, Favard C, Salah S, Brezin A, Vasseur V, Mauget-Faysse M (2020) Optical coherence tomography angiography analysis of retinal and choroidal vascular networks during acute, relapsing, and quiescent stages of macular toxoplasma retinochoroiditis. Biomed Res Int 2020:4903735. https://doi.org/10.1155/2020/4903735
Vezzola D, Allegrini D, Borgia A et al (2018) Swept-source optical coherence tomography and optical coherence tomography angiography in acquired toxoplasmic chorioretinitis: a case report. J Med Case Rep 12:358. https://doi.org/10.1186/s13256-018-1902-x
Kaufman HE (1960) The effect of corticosteroids on experimental ocular toxoplasmosis. Am J Ophthalmol 50:919–926. https://doi.org/10.1016/0002-9394(60)90344-5
Hofflin JM, Conley FK, Remington JS (1987) Murine model of intracerebral toxoplasmosis. J Infect Dis 155:550–557. https://doi.org/10.1093/infdis/155.3.550
Oray M, Ozdal PC, Cebeci Z, Kir N, Tugal-Tutkun I (2016) Fulminant Ocular toxoplasmosis: the hazards of corticosteroid monotherapy. Ocul Immunol Inflamm 24:637–646. https://doi.org/10.3109/09273948.2015.1057599
Holland GN, Lewis KG (2002) An update on current practices in the management of ocular toxoplasmosis. Am J Ophthalmol 134:102–114. https://doi.org/10.1016/s0002-9394(02)01526-x
Chodos JB, Habegger-Chodos HE (1961) The treatment of ocular toxoplasmosis with spiramycin. Arch Ophthalmol 65:401–409. https://doi.org/10.1001/archopht.1961.01840020403014
Funding
The authors declared that this study received no financial support.
Author information
Authors and Affiliations
Contributions
All co-authors have contributed to the study, and all have read and approved the final version of the manuscript. AOS and MK contributed to concept; AOS and MK contributed to design; FA, MK, BA and AOS contributed to data collection or processing; MK, BA, TT, AS and FA contributed to analysis or interpretation; FA, AOS, BA and MK contributed to literature search; FA, MK and AOS contributed to writing.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Animal research
There was no research on animals in this manuscript.
Consent to participate
Informed consent for participation was obtained from all study patients.
Consent to publish
Informed consent for publication was obtained from all study patients.
Ethics approval
This study was conducted in accordance with the tenets of the Helsinki Declaration after obtaining the approval of local ethics committee (Dokuz Eylül University: 2021/04-01).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Atas, F., Kaya, M., Toprak, . et al. Measurement of the active toxoplasma retinochoroiditis lesion size during the disease course with swept-source optical coherence tomography angiography: A retrospective image analysis. Int Ophthalmol 41, 4127–4135 (2021). https://doi.org/10.1007/s10792-021-01985-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-021-01985-w