Abstract
Purpose
To study the prevalence, antibiotic susceptibility profile, clinical outcomes and plasmid-mediated transfer of colistin resistance (CLR) among Gram-negative bacilli (GNB) isolates from different ocular infections.
Design
Prospective case–control study in eastern India.
Methods
Consecutive ocular samples with GNB isolates from clinically diagnosed cases of microbial keratitis, infectious endophthalmitis and orbital infections were included. Inclusion criteria were significant GNB growth from ocular samples and > 6 weeks follow-up. Clinical outcomes were determined by disease-specific criteria for each clinical group. Antibiotic susceptibility was tested by broth microdilution for colistin and Kirby–Bauer disc diffusion method for others. Plasmid detection for CLR genes mcr-1 and mcr-2 genes was done by standard protocols.
Results
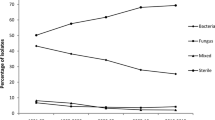
Sixty GNB isolates were studied. Overall prevalence of CLR (intrinsic plus acquired) was 40% (n = 24), acquired being 37.5% of CLR isolates (n = 9). The prevalence varied from 45.5% (10/22) and 45% (9/20) in microbial keratitis and infectious endophthalmitis, respectively, to 26.3% (5/19) in orbital infections. Clinical outcomes in CLR patients were significantly worse in microbial keratitis (p = 0.018) and orbital infections (p = 0.018), and comparable to colistin-susceptible ones (p = 0.77) in infectious endophthalmitis. CLR isolates had significantly higher resistance to Amikacin, Gentamicin and Ceftazidime but were susceptible to Piperacillin, Carbapenems and fluoroquinolones. Plasmids mcr-1 and mcr-2 were detected in 6.25% (n = 1) and 25%(n = 4), respectively, of the 16 tested isolates.
Conclusions
CLR is highly prevalent in ocular isolates and affects clinical outcomes. CLR isolates may still remain susceptible to Carbapenems, Piperacillin and fluoroquinolones. Plasmid mcr-1- and mcr-2-mediated CLR remains low in ocular infections.
Similar content being viewed by others
References
Asbell PA, Sanfilippo CM, Pillar CM et al (2015) Antibiotic resistance among ocular pathogens in the United States five-year results from the Antibiotic Resistance Monitoring in Ocular Microorganisms (ARMOR) surveillance study. JAMA Ophthalmol 133(12):1445–1455
Kunimoto DY, Das TP, Sharma S et al (1999) Microbiologic spectrum and susceptibility of isolates: part I. Postoperative endophthalmitis. Am J Ophthalmol 128(2):240–242
Sheng Y, Sun W, Gu Y et al (2011) Endophthalmitis after cataract surgery in China, 1995–2009. J Cataract Refract Surg 37:1715–1722
Irvine WD, Flynn HW Jr, Miller D et al (1992) Endophthalmitis caused by Gram negative organisms. Arch Ophthalmol 110:1450–1454
Lauren ML, Ly N, Anderson D et al (2010) Resurgence of colistin: a review of resistance, toxicity, pharmacodynamics and dosing. Pharmacotherapy 30(12):1279–1291
European Medicines Agency (2016) Updated advice on the use of colistin products in animals within the European Union: development of resistance and possible impact on human and animal health, pp 1–56
Jain R, Murthy SI, Motukupally SR, Jain M (2014) Use of topical colistin in multiple drug-resistant Pseudomonas aeruginosa bacterial keratitis. Cornea 33(9):923–927
Samant P, Ramugade S (2014) Successful use of intravitreal and systemic colistin in treating multidrug resistant Pseudomonas aeruginosa postoperative endophthalmitis. Indian J Ophthalmol 62(12):1167–1170
Dogra M, Sharma M, Katoch D, Dogra M (2018) Management of multi drug resistant endogenous Klebsiella pneumoniae endophthalmitis with intravitreal and systemic colistin. Indian J Ophthalmol 66(4):596–597
Taneja M, Senthil S, Paulose R et al (2016) Intravitreal colistin for multidrug resistant acute endophthalmitis following Descemet-stripping endothelial keratoplasty due to Klebsiella pneumoniae. JCRS Case Rep 4(3):52–56
Bialvaei A, Kafil HS (2015) Colistin, mechanisms and prevalence of resistance. Curr Med Res Opin 31(4):707–721
Karaiskos I, Giamarellou H (2014) Multidrug resistant and extensively drug resistant Gram-negative pathogens: current and emerging therapeutic approaches. Expert Opin Pharmacother 15(10):1351–1370
Loutet SA, Valvano MA (2011) Extreme antimicrobial peptide and polymyxin B resistance in the genus Burkholderia. Front Microbiol 2:159
Olaitan AO, Morand S, Rolain JM (2014) Mechanisms of polymyxin resistance: acquired and intrinsic resistance in bacteria. Front Microbiol 5(643):1–18
Falagas ME, Rafailidis PI, Matthaiou DK (2010) Resistance to polymyxins: mechanisms, frequency and treatment options. Drug Resist Updates 13:132–138
Jayol A, Poirel L, Dortet L, Nordmann P (2016) National survey of colistin resistance among carbapenemase-producing Enterobacteriaceae and outbreak caused by colistin-resistant OXA-48-producing Klebsiella pneumoniae, France, 2014. Euro Surveill 21(37):30339
Terveer EM, Nijhuis R, Crobach M et al (2017) Prevalence of colistin resistance gene (mcr-1) containing Enterobacteriaceae in faeces of patients attending a tertiary care hospital and detection of a mcr-1 containing, colistin susceptible E. coli. PLoS One 12(6):e0178598
Arjun R, Gopalakrishnan R, Nambi PS et al (2017) A study of 24 patients with colistin-resistant Gram-negative isolates in a tertiary care hospital in South India. Indian J Crit Care Med 21(5):317–321
Fernandes M, Vira D, Medikonda R, Kumar N (2015) Extensively and pandrug resistant Pseudomonas aeruginosa keratitis: clinical features, risk factors and outcome. Graefes Arch Clin Exp Ophthalmol 254(2):315–322
Sanghi S, Pathengay A, Jindal A et al (2014) Acute-onset post-operative endophthalmitis caused by multidrug-resistant Klebsiella pneumoniae. Clin Ophthalmol 8:1783–1785
Jones DB, Liesegang TJ, Robinson NM (1994) Laboratory diagnosis of ocular infections. Cumitech 13A. Am Soc Microbio 31
Recommendations for MIC determination of colistin (polymyxin E) as recommended by the joint CLSI-EUCAST Polymyxin Breakpoints Working Group (2016). Published on www.eucast.org. Accessed July 2017
Cavaco L, Mordhorst H, Hendriksen R (2016) PCR for plasmid-mediated colistin resistance genes, mcr-1 and mcr-2 (multiplex). Laboratory protocol. European Union Reference Laboratory. Antimicrobial Resistance. Version 2
Prim N, Turbau M, Rivera A et al (2017) Prevalence of colistin resistance in clinical isolates of Enterobacteriaceae: a four-year cross-sectional study. J Infect. https://doi.org/10.1016/j.jinf.2017.09.008
Poirel L, Jayol A, Nordmann P (2017) Polymyxins: antibacterial activity, susceptibility, testing and resistance mechanisms encoded by plasmids or chromosomes. Clin Microbiol Rev 30(2):557–597
Veeraraghavan B, Jesudason MR, Prakasah JA et al (2018) Antimicrobial susceptibility profiles of Gram-negative bacteria causing infections collected across India during 2014–2016: study for monitoring antimicrobial resistance trend report. Indian J Med Microbiol 36:32–36
Ramesh N, Prasanth M, Ramkumar S et al (2016) Colistin susceptibility of Gram negative clinical isolates from Tamil Nadu, India. Asian Biomed 10(1):35–39
Liu YY, Wang W, Walsh TR et al (2016) Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis 16:161–168
Magiorakos AP, Srinivasan A, Carey RB et al (2012) Multidrug resistant, extensively drug resistant and pandrug resistant bacteria: an international expert proposal for interim standard definition for acquired resistance. Clin Microbiol Infect 18(3):268–281
Jindal A, Pathengay A, Khera M et al (2013) Combined ceftazidime and amikacin resistance among Gram-negative isolates in acute-onset post-operative endophthalmitis: prevalence, antimicrobial susceptibilities and visual outcome. J Ophthalmic Inflamm Infect 3:62. https://doi.org/10.1186/1869-5760-3-62
Norberg P, Bergstrom M, Jethava V et al (2011) The IncP-1 plasmid backbone adapts to different host bacterial species and evolves through homologous recombination. Nat Commun 2:268. https://doi.org/10.1038/ncomms1267
Rebelo AR, Bortolaia V, Kjeldgaard JS et al (2018) Multiplex PCR for detection of plasmid-mediated colistin resistance determinants, mcr-1, mcr-2, mcr-3, mcr-4 and mcr-5 for surveillance purposes. Euro Surveill. https://doi.org/10.2807/1560-7917.ES.2018.23.6.17-00672
Exner M, Bhattacharya S, Christiansen B et al (2017) Antibiotic resistance: what is so special about multi-drug resistant Gram-negative bacteria? GMS Hyg Infect Control 12. ISSN2196-5226
Acknowledgements
We thank to Aparajita Mullick, Silpa Priyadarshini, Manas Ranjan Barik, Savitri Sharma, Shivaji Sisinthy, Prity Sahay, Swati Shradhhanjali—Ocular Microbiology and Molecular Biology, L. V. Prasad Eye Institute, Bhubaneswar and Hyderabad.
Funding
This study was funded by HERF (Hyderabad Eye Research Foundation).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors report any conflict of interest.
Ethical approval
Institutional ethics committee approval (2017-91-IM-17) was taken and tenets of Declaration of Helsinki were adhered to. This study does not contain any studies with animals, performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants, whose clinical information had been included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mitra, S., Basu, S., Rath, S. et al. Colistin resistance in Gram-negative ocular infections: prevalence, clinical outcome and antibiotic susceptibility patterns. Int Ophthalmol 40, 1307–1317 (2020). https://doi.org/10.1007/s10792-020-01298-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-020-01298-4