Abstract
Purpose
To evaluate the visual and anatomical outcomes of intravitreal ranibizumab for diabetic macular edema (DME) in the healthcare system of Taiwan.
Methods
A total of 39 eyes from 39 patients were retrospectively enrolled in the study. All eyes that fulfilled the key criteria, including a baseline vision between 20 and 70 ETDRS letters and a minimum central macular thickness (CMT) of 300 µm, had at least 3 monthly loading injections of ranibizumab in a year. Macular laser or posterior subtenon injections of triamcinolone acetonide (PSTA) could be performed as supplementary treatments following loading injections. Primary outcomes include best-corrected visual acuity and CMT.
Results
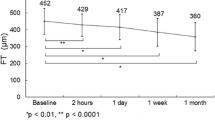
Patients’ vision improved from 46.5 ± 15.3 letters at baseline to 51.4 ± 16.6 letters at 12 months (p = 0.031). Mean CMT at baseline was 406 ± 105 µm, which decreased to 329 ± 108 µm (p = 0.002). At 12 months, 44.4% of eyes with total injection number < 5 and 42.9% with injection number ≥ 5 achieved a gain in vision that was 10 letters or more. A total of 5 injections or more did not lead to a better visual gain in comparison with only 3–4 injections (p = 0.71), and both had similar number of supplementary treatments (p = 0.43). Monthly reinjections of ranibizumab resulted in a lower likelihood of visual loss of 10 or 15 letters (p = 0.019 and 0.015, respectively, adjusted for age, baseline vision, severity of diabetic retinopathy and the presence of previous treatments); however, supplementary macular lasers, PSTA or ranibizumab without monthly reinjections did not (all p > 0.05). The average number of injections was 4.3 ± 1.0.
Conclusion
Treatment for DME with at least three monthly ranibizumab loading injections, with or without other supplementary treatments, is effective at 12 months thereafter. Two monthly reinjections of ranibizumab, while not significantly increasing vision, may have a role in preventing visual loss.





Similar content being viewed by others
Abbreviations
- DME:
-
Diabetic macular edema
- VEGF:
-
Vascular endothelial growth factor
- ETDRS:
-
Early Treatment Diabetic Retinopathy Study
- BCVA:
-
Best-corrected visual acuity
- CMT:
-
Central macular thickness
- PSTA:
-
Posterior subtenon injection of triamcinolone acetonide
References
Lee R, Wong TY, Sabanayagam C (2015) Epidemiology of diabetic retinopathy, diabetic macular edema and related vision loss. Eye Vis (Lond) 2:17. https://doi.org/10.1186/s40662-015-0026-2
Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O’Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY, Meta-Analysis for Eye Disease Study G (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35(3):556–564. https://doi.org/10.2337/dc11-1909
Yamagishi S, Ueda S, Matsui T, Nakamura K, Okuda S (2008) Role of advanced glycation end products (AGEs) and oxidative stress in diabetic retinopathy. Curr Pharm Des 14(10):962–968
Yamagishi S, Matsui T (2011) Advanced glycation end products (AGEs), oxidative stress and diabetic retinopathy. Curr Pharm Biotechnol 12(3):362–368
Stewart MW (2016) Treatment of diabetic retinopathy: recent advances and unresolved challenges. World J Diabetes 7(16):333–341. https://doi.org/10.4239/wjd.v7.i16.333
Boyer DS, Yoon YH, Belfort R Jr, Bandello F, Maturi RK, Augustin AJ, Li XY, Cui H, Hashad Y, Whitcup SM, Ozurdex MSG (2014) Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 121(10):1904–1914. https://doi.org/10.1016/j.ophtha.2014.04.024
Bakri SJ, Kaiser PK (2005) Posterior subtenon triamcinolone acetonide for refractory diabetic macular edema. Am J Ophthalmol 139(2):290–294. https://doi.org/10.1016/j.ajo.2004.09.038
Ishibashi T, Li X, Koh A, Lai TY, Lee FL, Lee WK, Ma Z, Ohji M, Tan N, Cha SB, Shamsazar J, Yau CL, Group RS (2015) The REVEAL study: ranibizumab monotherapy or combined with laser versus laser monotherapy in Asian Patients with diabetic macular edema. Ophthalmology 122(7):1402–1415. https://doi.org/10.1016/j.ophtha.2015.02.006
Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, Sutter F, Simader C, Burian G, Gerstner O, Weichselberger A, Group RS (2011) The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 118(4):615–625. https://doi.org/10.1016/j.ophtha.2011.01.031
Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, Mitchell P, Sharp D, Wolf-Schnurrbusch UE, Gekkieva M, Weichselberger A, Wolf S (2010) Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care 33(11):2399–2405. https://doi.org/10.2337/dc10-0493
Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L, Schlottmann PG, Rundle AC, Zhang J, Rubio RG, Adamis AP, Ehrlich JS, Hopkins JJ, Ride, Group RR (2013) Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology 120(10):2013–2022. https://doi.org/10.1016/j.ophtha.2013.02.034
Pearce I, Banerjee S, Burton BJ, Chakravarthy U, Downey L, Gale RP, Gibson J, Pagliarini S, Patel J, Sivaprasad S, Andrews C, Brittain C, Warburton J, Group RS (2015) Ranibizumab 0.5 mg for diabetic macular edema with bimonthly monitoring after a phase of initial treatment: 18-month, multicenter, phase IIIB RELIGHT study. Ophthalmology 122(9):1811–1819. https://doi.org/10.1016/j.ophtha.2015.05.038
Ghanchi F, Hazel CA (2016) South Asian diabetic macular oedema treated with ranibizumab (ADMOR)-real-life experience. Eye (Lond) 30(1):133–138. https://doi.org/10.1038/eye.2015.209
Ross EL, Hutton DW, Stein JD, Bressler NM, Jampol LM, Glassman AR, Diabetic Retinopathy Clinical Research N (2016) Cost-effectiveness of aflibercept, bevacizumab, and ranibizumab for diabetic macular edema treatment: analysis from the diabetic retinopathy clinical research network comparative effectiveness trial. JAMA Ophthalmol 134(8):888–896. https://doi.org/10.1001/jamaophthalmol.2016.1669
Patrao NV, Antao S, Egan C, Omar A, Hamilton R, Hykin PG, Sivaprasad S, Rajendram R, Moorfields Diabetic Macular Edema Study G (2016) Real-world outcomes of ranibizumab treatment for diabetic macular edema in a United Kingdom National Health Service setting. Am J Ophthalmol. https://doi.org/10.1016/j.ajo.2016.09.002
Sophie R, Lu N, Campochiaro PA (2015) Predictors of functional and anatomic outcomes in patients with diabetic macular edema treated with ranibizumab. Ophthalmology 122(7):1395–1401. https://doi.org/10.1016/j.ophtha.2015.02.036
Bressler SB, Qin H, Beck RW, Chalam KV, Kim JE, Melia M, Wells JA 3rd, Diabetic Retinopathy Clinical Research N (2012) Factors associated with changes in visual acuity and central subfield thickness at 1 year after treatment for diabetic macular edema with ranibizumab. Arch Ophthalmol 130(9):1153–1161. https://doi.org/10.1001/archophthalmol.2012.1107
Elman MJ, Ayala A, Bressler NM, Browning D, Flaxel CJ, Glassman AR, Jampol LM, Stone TW, Diabetic Retinopathy Clinical Research N (2015) Intravitreal Ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: 5-year randomized trial results. Ophthalmology 122(2):375–381. https://doi.org/10.1016/j.ophtha.2014.08.047
Zhang L, Wang W, Gao Y, Lan J, Xie L (2016) The efficacy and safety of current treatments in diabetic macular edema: a systematic review and network meta-analysis. PLoS ONE 11(7):e0159553. https://doi.org/10.1371/journal.pone.0159553
Bressler SB, Glassman AR, Almukhtar T, Bressler NM, Ferris FL, Googe JM Jr, Gupta SK, Jampol LM, Melia M, Wells JA 3rd, Diabetic Retinopathy Clinical Research N (2016) Five-year outcomes of ranibizumab with prompt or deferred laser versus laser or triamcinolone plus deferred ranibizumab for diabetic macular edema. Am J Ophthalmol 164:57–68. https://doi.org/10.1016/j.ajo.2015.12.025
Rahimy E, Shahlaee A, Khan MA, Ying GS, Maguire JI, Ho AC, Regillo CD, Hsu J (2016) Conversion to aflibercept after prior anti-VEGF therapy for persistent diabetic macular edema. Am J Ophthalmol 164(118–127):e112. https://doi.org/10.1016/j.ajo.2015.12.030
Lee JH, Lee WK, Kim SE (2016) Short-term outcomes of switching to ranibizumab therapy for diabetic macular edema in patients with persistent fluid after bevacizumab therapy. J Ocul Pharmacol Ther. https://doi.org/10.1089/jop.2016.0074
Hanhart J, Chowers I (2015) Evaluation of the response to ranibizumab therapy following bevacizumab treatment failure in eyes with diabetic macular edema. Case Rep Ophthalmol 6(1):44–50. https://doi.org/10.1159/000375230
Chong V (2012) Biological, preclinical and clinical characteristics of inhibitors of vascular endothelial growth factors. Ophthalmologica 227(Suppl 1):2–10. https://doi.org/10.1159/000337152
Binder S (2012) Loss of reactivity in intravitreal anti-VEGF therapy: tachyphylaxis or tolerance? Br J Ophthalmol 96(1):1–2. https://doi.org/10.1136/bjophthalmol-2011-301236
Menchini U, Bandello F, De Angelis V, Ricci F, Bonavia L, Viola F, Muscianisi E, Nicolo M (2015) Ranibizumab for visual impairment due to diabetic macular edema: real-world evidence in the Italian population (PRIDE study). J Ophthalmol 2015:324841. https://doi.org/10.1155/2015/324841
Brynskov T, Laugesen CS, Sorensen TL (2013) Intravitreal ranibizumab for diabetic macular oedema: 1-year experiences in a clinical setting. Acta Ophthalmol 91(3):e243–e244. https://doi.org/10.1111/aos.12014
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no any competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This research adhered to the tenets of the Declaration of Helsinki, and institutional review board (IRB) approval was obtained from the IRB of Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Tsai, MJ., Hsieh, YT. & Peng, YJ. Real-life experience of ranibizumab for diabetic macular edema in Taiwan. Int Ophthalmol 39, 1511–1522 (2019). https://doi.org/10.1007/s10792-018-0970-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-018-0970-7