Abstract
Purpose
To investigate the changes in choroidal thickness (ChT) following panretinal photocoagulation (PRP) for diabetic retinopathy (DR) and compare ChT in relation to DR severity.
Methods
Thirty-two eyes [19 eyes with proliferative DR (PDR) and 13 eyes with severe nonproliferative DR (NPDR)] for which PRP was necessary were analyzed. ChT was measured before PRP and at 1, 3, and 6 months after PRP using the swept-source optical coherence tomography. ChT of the 61 eyes matched with the PDR patients for the mean age and axial length was also measured and statistically compared in relation to severity.
Results
The central field ChT before PRP treatment was 268.6 ± 104.5 µm (mean ± standard deviation) and was significantly decreased at 1, 3, and 6 months after PRP (254.5 ± 105.3, 254.2 ± 108.2, and 248.1 ± 101.8 µm, respectively, P < 0.0001). The central field ChT of severe NPDR (323.2 ± 61.3 µm) was significantly thicker than that of normal (248.3 ± 70.7 µm) and mild to moderate NPDR (230.0 ± 70.3 µm, P = 0.0455 and 0.0099, respectively). Moreover, the central field ChT of PDR (307.3 ± 84.1 µm) was significantly thicker than of mild to moderate NPDR (P = 0.0169).
Conclusion
ChT significantly decreased after PRP, which continued for at least 6 months after treatment. ChT of severe NPDR and PDR was significantly thicker than that of mild to moderate NPDR. ChT of patients with DR was changed according to the treatment and severity of DR.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic retinopathy (DR) is still a leading cause of blindness in working populations in both developing or advanced countries [1]. Panretinal photocoagulation (PRP) is an established treatment for this serious ocular disease [2], and it has been performed on patients with both severe nonproliferative DR (NPDR) and proliferative DR (PDR) [3]. Diabetes mellitus is a disease that affects the retina and can also cause systemic microcirculatory disorders. When such microvascular complications progress, the risk of developing macroangiopathy also increases [4, 5]. In particular, diabetes impairs the ocular circulation by causing changes in the retinal blood vessels and likely affects choroidal blood flow. There are many unknown factors regarding the effects of DR on choroid and the mechanisms by which PRP changes the choroid.
Since Spaide et al. [6] reported a noninvasive evaluation of choroidal thickness (ChT) in normal volunteers using enhanced depth imaging optical coherence tomography (EDI-OCT) in 2008, several studies on ChT have been reported using EDI method [7–13]. As for ChT in patients with DR, Regatieri and Unsal [7, 8] reported that ChT in patients with diabetic macula edema and PRP-treated PDR were thinner than in normal subjects. In addition, Kim et al. [9] reported that ChT in patients with PDR was significantly increased compared with patients with mild or moderate NPDR, whereas ChT in PRP-treated eyes was thinner.
Several studies [10–13] have reported longitudinal changes in ChT before and after the PRP procedure; however, the results of the studies are still controversial; ChT was found to be increased [10, 11], decreased [12], or increased then decreased [13].
However, measuring ChT in fovea by the pinpoint measurement in EDI is likely influenced by the increase or decrease in the regional ChT as well as the irregularity of the choroidoscleral border [14]. Additionally, measurement of retinal thickness of the central subfield around the fovea provided better reproducibility than did the measurement of the center point [15]. Thus, a 3D volumetric raster scan by swept-source optical coherence tomography (SS-OCT) is considered to be a superior method for measuring the ChT.
The first aim of the study was to clarify the longitudinal ChT changes in PRP-treated eyes. The second aim was to compare ChT among patients with DR as well as normal subjects using SS-OCT.
Methods
Patients
Patients with severe NPDR or PDR for whom PRP was considered necessary and performed between August 2014 and March 2015 were enrolled in this study. A total of 70 eyes of 40 patients with severe NPDR and 56 eyes of 36 patients with PDR were enrolled for this study. To compare the severity of DR and ChT, healthy subjects and diabetic patients without DR (noDR), mild to moderate NPDR (m–m NPDR), and severe NPDR and PDR without PRP were enrolled. This study was approved by the Institutional Review Board of Saneikai Tsukazaki Hospital and performed in accordance with the Declaration of Helsinki. Written, informed consent to participate in the study was obtained from all participants. All patients underwent an eye examination including best-corrected visual acuity, slit lamp microscopy, and fundus photography (Optos 200Tx, Optos Inc., Scotland, UK). Visual acuity was presented in logMAR, and the axial length was also measured using IOL Master (Carl Zeiss Meditec, Jena, Germany). Fluorescein angiography (FA) was performed to accurately determine the severity in severe NPDR and PDR eyes. The severity of the retinopathy was based on the classification proposed by the Global Diabetic Retinopathy Project Group [16].
Eyes with a retinal disease other than DR, glaucoma, ocular trauma, history of uveitis, and turbid ocular media that can obscure the border of the choroid in optical coherence tomography (OCT) were excluded. Eyes that had undergone cataract surgery in the last 12 months were also excluded. For eyes with DR, only treatment-naive eyes were included, and those with a history of photocoagulation, vitreous injection of anti-VEGF drugs, and vitreous surgery were excluded. Eyes treated with PRP using a pattern scan laser were excluded.
The right eye was enrolled in the study; if the right eye met any of the exclusion criteria, the left eye was enrolled. For bilaterally PRP-treated eyes with different severity, each eye was enrolled according to its severity.
Treatment protocol
PRP was performed over four sessions every other week. The procedure was carried out in the following order: (1) superior; (2) nasal; (3) inferior; and (4) temporal. The spot size was set at 200–250 µm with an exposure duration of 0.2–0.3 s. A panfundus contact lens (Ocular Mainster PRP 165, Ocular, Bellevue, WA, USA) was worn, and the total number of exposures was between 900 and 1900. The multicolor photocoagulation laser device (NOVUS Varia, Lumenis, UT, USA) with a wavelength of 532 (green) or 561 (yellow) nm was used. According to the ETDRS protocol [17], the laser power was adjusted to induce gray–white spots.
For eyes that had fovea-involving macular edema before the PRP treatment, or when macular edema was found during treatment, 20-mg sub-Tenon injection of triamcinolone acetonide (STTA) was administered. The decision to treat with STTA was made at the discretion of an experienced ophthalmologist.
Swept-source optical coherence tomography
The macular area (6 × 6 mm) was measured using SS-OCT (DR1 OCT-1; Topcon, Tokyo, Japan). The SS-OCT system is a swept-source laser device that can provide a scanning speed of 100,000 A-scans per second with a tuning range of approximately 100 nm centered at the 1-µm wavelength region with an imaging depth of 2.6 mm. The SS-OCT measurements were performed by trained examiners after inducing pupil dilation. Using a 3D volumetric raster scan protocol, 3D volumetric data were obtained in 0.8 s, and each 3D scan covered a 6 × 6 mm area centered on the fovea with 512 A-scans × 256 B-scans. To improve the quality of the images, four consecutive B-scan images of the same area were averaged [14].
Measurements of choroidal thickness
All patients who scheduled for PRP treatment underwent SS-OCT examinations before and after each session at 1, 3, and 6 months after PRP. Additionally, the patients underwent the SS-OCT examination at the time of the initial visit or at the regular visit and their DR severity and ChT were compared. With a set of 64 B-scan images obtained by averaging each of the four consecutive B-scans, a ChT map was created using semiautomatic segmentation. ChT was measured as the distance between the outer border of the retinal pigment epithelium and the inner surface of the choroidal-scleral border. However, semiautomatic segmentation does not always accurately detect the choroidal-scleral border. Therefore, all 64 B-scan images were manually corrected by certified experienced orthoptists using built-in software. The interobserver reproducibility of the ChT measurements obtained by this manual segmentation has previously been reported by our team [14], and the intraclass correlation coefficient (ICC) of the ChT measurements by two observers was extremely favorable (between 0.990 and 0.999).
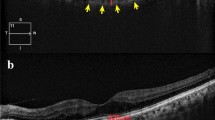
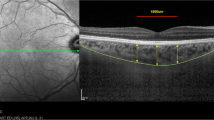
ChT is automatically divided into sections of the ETDRS map, and the mean ChT of each section is automatically calculated and presented by the software. The inner and outer rings are 3 and 6 mm in diameter, respectively, and each ring is divided into four parts. The mean central field choroidal thickness (CFChT) was defined as the mean ChT of the center area spanning a 1 mm diameter on the ETDRS map (Fig. 1A).
Choroidal thickness (ChT) measurements using SS-OCT. A The mean central field choroidal thickness (CFChT) was defined as the mean ChT of the center area spanning a 1 mm diameter on the ETDRS map (white arrow). B The subfoveal choroidal thickness (SFChT), which is the distance between the outer RPE border and the inner surface of the choroidal–scleral border, was automatically calculated on the images that went through the subfovea (white arrowhead)
In addition, to obtain the subfoveal choroidal thickness (SFChT) using the same point measurements as the EDI method from the scan images obtained to measure CFChT, the subfovea was manually marked on the images that went through the subfovea. Subsequently, the distance between the outer retinal pigment epithelium border and the inner surface of the choroidal-scleral border was automatically calculated and statistically compared (Fig. 1B).
Statistical analyses
The statistical analyses were performed using JMP version 10.0 software package (SAS Institute Inc., Cary, NC, USA). A comparison of the duration of DR was performed using a one-way analysis of variance (ANOVA) followed by a Tukey’s HSD test. A comparison of ChT at each time point was performed first by repeated measures one-way ANOVA, and a comparison between groups was performed by Tukey’s HSD test. Additionally, a two-way repeated measures ANOVA was used to compare between STTA-treated and untreated eyes. A P value of less than 0.05 was considered statistically significant.
Results
The characteristics of the PRP-treated patients are presented in Table 1. Ninety-four eyes of 53 patients were excluded as they did not meet the inclusion criteria. Finally, 32 eyes from 23 subjects (6 females and 17 males) completed the study and data were subsequently analyzed.
The mean age (SD) of the PRP patients was 55.6 (±12.8) years (range: 37–79 years). The mean axial length (SD) was 23.6 (±0.98) mm (range: 21.7–26.0 mm), and the mean known disease duration of diabetes mellitus (SD) was 13.5 (±11.8) years (range: 1–43 years). Based on the international classification of DR, 19 eyes were considered PDR and 13 eyes were severe NPDR. The mean (SD) logMAR visual acuity at baseline was 0.06 (±0.19) (range: −0.18 to 0.52), and the logMAR visual acuity at 6 months was 0.09 (±0.23) (range: −0.18 to 0.82).
Longitudinal ChT changes in PRP
CFChT before treatment was 268.6 ± 104.3 µm and at 1, 3, and 6 months was 254.5 ± 105.3, 254.2 ± 108.2, and 248.1 ± 101.8 µm, respectively. The result of the repeated measures one-way ANOVA was P < 0.0001, and the group comparison using a Tukey’s HSD test showed that the mean CFChT was significantly decreased at all post-treatment time points compared with the pretreatment value (all P < 0.005) (Fig. 2).
Changes in the central field choroidal thickness (CFChT) after panretinal photocoagulation (PRP). The mean CFChT after PRP at 1, 3, and 6 months was clearly decreased compared with the baseline values. Asterisk indicates the significant difference between the measurements points by Tukey’s HSD (P < 0.005). Error bars represent standard error of the mean
Multiple comparisons tests showed no significant difference between 1 and 3 months and between 3 and 6 months (P = 0.9998 and 0.1392, respectively). Moreover, six eyes received STTA during the study period. No statistically significant difference was found for the mean CFChT between the STTA-treated eyes (N = 6) and eyes without STTA treatment (N = 26) (multivariate ANOVA, P = 0.7921).
SFChT was 268.4 ± 102.9 µm before treatment and 253.4 ± 103.1, 253.8 ± 107.1, and 252.9 ± 110.5 µm at 1, 3, and 6 months after treatment, respectively. The results of the repeated measures one-way ANOVA were P = 0.0002, and the group comparison using the Tukey’s HSD test showed that the mean SFChT was significantly decreased at all post-treatment time points compared with the pretreatment value (all P < 0.005) (Fig. 3).
Changes in the subfoveal choroidal thickness (SFChT) after panretinal photocoagulation (PRP). The mean SFChT after PRP at 1, 3, and 6 months was clearly decreased compared with the baseline values. Asterisk indicates the significant difference between the measurements points by Tukey’s HSD (P < 0.005). Error bars represent standard error of the mean
Multiple comparisons tests showed no significant difference between 1 and 3 months and between 3 and 6 months (P = 0.9998 and 0.9532, respectively).
Comparison of ChT among the different groups of DR severity and normal subjects
Table 2 shows the characteristics of the patients included in the comparison of ChT with severity of DR. The mean CFChT of the eyes for each severity matched for the mean age and axial length with the untreated PDR patients was as follows: normal, 248.3 ± 70.7; noDR, 250.2 ± 55.4; m–m NPDR, 230.0 ± 70.3; severe NPDR, 323.2 ± 61.3; and PDR, 307.3 ± 84.1.
ChT of the PDR group was significantly thicker than that of the m–m NPDR group. Moreover, CFChT of the severe NPDR group was significantly thicker than that of the normal and m–m NPDR groups (P < 0.005) (Fig. 4).
The mean SFChT of the eyes for each severity group was as follows: normal, 243 ± 71.4; noDR, 251.3 ± 61.9; m–m NPDR, 227.1 ± 71.3; severe NPDR, 323.1 ± 66.0; and PDR, 301.7 ± 80.8. In addition, SFChT of the PDR group was significantly thicker than that of the m–m NPDR group; SFChT of the severe NPDR group was significantly thicker than that of the normal and m–m NPDR groups (Fig. 5). The results of CFChT and SFChT were extremely similar.
Discussion
In the current study, we confirmed that PRP treatment causes a decrease in ChT, which continues for up to 6 months. As photocoagulation scarring becomes enlarged over time [18], changes in ChT are also likely to continue over a long period. The choroid consists of abundant vascular components and may be greatly influenced by changes in its blood flow. Our observation period of 6 months is appropriate because ocular blood flow velocities are stable from 6 months to 2 years following PRP [19].
According to the early longitudinal studies on ChT after PRP [10–13], SFChT increased after 1 week [10, 13]. Cho et al. [10] speculated that increases in ChT are due to increased blood flow due to vasodilation or choroidal effusion induced by the laser, which damages the peripheral choriocapillaris, consequently decreasing the peripheral choroidal blood flow. As a result, redistribution of blood flow occurs, and the choroidal blood flowing around the foveal centralia increases, resulting in an increase in SFChT. However, in the present study, ChT at 1 week after PRP was not measured and thus cannot be directly compared.
Meanwhile, some reports showed that SFChT was decreased at 1 month [12, 13] and 3 months after PRP [13], which suggested that ChT tends to decrease over time. Considering these findings together with the data from our present study, although ChT may increase in the short term, it tends to decrease over the long term.
Zhang et al. [13] presented three hypotheses regarding the observed decreases in ChT: (1) thermal damage by photocoagulation spreads to the choroid which consequently interferes with choroidal reperfusion or reorganization, and results in a reduction in ChT; (2) PRP damages the RPE and decreases VEGF secretion which results in decreased dilation and permeability of the choroidal vessels; (3) the outer retina is destroyed and the hypoxic inner retina comes closer to the highly saturated choriocapillaris, which then improves the hypoxic state. Autoregulation decreases the choroidal blood flow and causes a decrease in ChT.
However, Zhu et al. [11] reported a significant increase in SFChT at 1 and 3 months after PRP, revealing completely different results from other studies. They argued [11] that a thinner ChT around the photocoagulated area causes a reduction in the peripheral choroidal blood flow, resulting in a redistribution of blood supply to the fovea that consequently increases SFChT. In our study as well as others, a conventional laser was used to perform PRP. In contrast, Zhu et al. [11] used a pattern scan laser PRP. It has been reported that the laser scarring from a pattern scan laser reduces rather than expands in size, and the reduction can be up to 35% [20]. In addition, a study which histopathologically compared the scars by both a conventional and pattern scan laser reported that conventional laser scar lesions exhibited both outer and inner nuclear layer loss. In contrast, pattern scan laser scar lesions exhibited only outer nuclear layer loss, and the inner nuclear layer was preserved [21]. This difference may have contributed to the inconsistent results between these studies.
Previous studies have reported that ChT negatively correlates with age and axial length [22, 23]. Therefore, in this study, the mean CFChT was statistically compared with the data matched with PDR patients for age and axial length. The results indicate that the mean ChT of the severe NPDR and PDR groups was significantly thicker than that of the m–m NPDR group. Moreover, the mean ChT of the severe NPDR group was also significantly thicker than that of the normal group. These findings illustrate that as the severity worsens, ChT also increases. These findings are supported by Kim et al. [9], who found that ChT significantly increased as the severity worsened from mild/moderate NPDR to PDR. We suspected that the same point measurements as EDI-OCT might be easily affected by the local increase or decrease in ChT, as well as the irregularity of the choroidoscleral border; however, the values were almost identical to the mean values obtained within the 1-mm center area (CFChT). As the choroid is thickest at the fovea centralis in DR [7–9], this seems to be the reason why there was no difference observed between CFChT and SFChT.
Increases in ChT as the severity of retinopathy worsens are likely caused by an increase in vascular endothelial growth factor (VEGF). DR causes retinal nonperfusion, and VEGF is produced. VEGF is induced by hypoxia and results in elevated intraocular VEGF levels [24–26]. One study found that ChT was thinner when patients were treated with a combination of laser treatment and anti-VEGF therapy than when they were treated with laser treatment alone [27]. Such findings suggest that VEGF causes the choroid to thicken.
As ChT tends to increase the severity of DR and PRP significantly decreases ChT, a positive correlation between ChT and DR activity or intraocular VEGF level may exist. Based on this assumption, the decrease in ChT following PRP and the continued decrease for at least 6 months observed in this study may be associated with a decline in the retinopathy symptoms. Therefore, ChT may be a useful indicator for controlling the progression of DR.
Due to the small sample size of our study, the possibility of biased results cannot be ruled out. The reliability of our results should be further validated by studying a larger number of cases. In our study, there were no statistical differences in ChT between the STTA-treated eyes and those that did not receive treatment; however, there was a study which reported a decrease in ChT in DME eyes following intravitreal triamcinolone acetonide [28]. Thus, this may have influenced our study results. Additionally, having a longer follow-up period may help to clarify the relationship between ChT and severity of retinopathy.
Conclusion
ChT significantly decreased following PRP, and the decline continued for at least 6 months after treatment. ChT of patients with severe NPDR and PDR was significantly thicker than that with m–m NPDR patients. ChT of patients with DR changed according to the treatment and severity of DR.
References
Ruta LM, Magliano DJ, Lemesurier R, Taylor HR, Zimmet PZ, Shaw JE (2013) Prevalence of diabetic retinopathy in Type 2 diabetes in developing and developed countries. Diabet Med 30:387–398
The Diabetic Retinopathy Study Research Group (1981) Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. Ophthalmology 88:583–600
Early Treatment Diabetic Retinopathy Study Research Group (1991) Early photocoagulation for diabetic retinopathy. ETDRS Report Number 9. Ophthalmology 98(Suppl 5):766–785
Fuller JH, Stevens LK, Wang SL (2001) Risk factors for cardiovascular mortality and morbidity: the WHO Mutinational Study of Vascular Disease in Diabetes. Diabetologia 44(Suppl 2):S54–S64
Adler AI, Stevens RJ, Manley SE, Bilous RW, Cull CA, Holman RR, UKPDS GROUP (2003) Development and progression of nephropathy in type 2 diabetes: the United Kingdom Prospective Diabetes Study (UKPDS 64). Kidney Int 63(1):225–232
Spaide RF, Koizumi H, Pozonni MC (2008) Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol 46:496–500
Regatieri CV, Branchini L, Carmody J, Fujimoto JG, Duker JS (2012) Choroidal thickness in patients with diabetic retinopathy analyzed by spectral-domain optical coherence tomography. Retina 32:563–568
Unsal E, Eltutar K, Zirtiloğlu S, Dinçer N, Ozdoğan Erkul S, Güngel H (2014) Choroidal thickness in patients with diabetic retinopathy. Clin Ophthalmol 8:637–642
Kim JT, Lee DH, Joe SG, Kim JG, Yoon YH (2013) Changes in choroidal thickness in relation to the severity of retinopathy and macular edema in type II diabetic patients. Invest Ophthalmol Vis Sci 54:3378–3384
Cho GE, Cho HY, Kim YT (2013) Change in subfoveal choroidal thickness after argon laser panretinal photocoagulation. Int J Ophthalmol 6:505–509
Zhu Y, Zhang T, Wang K, Xu G, Huang X (2015) Changes in choroidal thickness after panretinal photocoagulation in patients with type 2 diabetes. Retina 35:695–703
Lee SH, Kim J, Chung H, Kim HC (2014) Changes of choroidal thickness after treatment for diabetic retinopathy. Curr Eye Res 39:736–744
Zhang Z, Meng X, Wu Z, Zou W, Zhang J, Zhu D et al (2015) Changes in choroidal thickness after panretinal photocoagulation for diabetic retinopathy: a 2-week longitudinal study. Invest Ophthalmol Vis Sci 56:2631–2638
Nagasawa T, Mitamura Y, Katome T, Shinomiya K, Naito T, Nagasato D et al (2013) Macular choroidal thickness and volume in healthy pediatric individuals measured by swept-source optical coherence tomography. Invest Ophthalmol Vis Sci 54:7068–7074
Diabetic Retinopathy Clinical Research Network, Krzystolik MG, Strauber SF, Aiello LP, Beck SF, Berger BB et al (2007) Reproducibility of macular thickness and volume using zeiss optical coherence tomography in patients with diabetic macular edema. Ophthalmology 114:1520–1525
Wilkinson CP, Ferris FL III, Klein RE, Lee PP, Agardh CD, Davis M, Global Diabetic Retinopathy Project Group et al (2003) Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 110:1677–1682
The Early Treatment Diabetic Retinopathy Study Research Group (1987) Techniques for scatter and local photocoagulation treatment of diabetic retinopathy: Early Treatment Diabetic Retinopathy Study Report No. 3. Int Ophthalmol Clin 27:254–264
Maeshima K, Utsugi-Sutoh N, Otani T, Kishi S (2004) Progressive enlargement of scattered photocoagulation scars in diabetic retinopathy. Retina 24:507–511
Mendivil A, Cuartero V (1996) Ocular blood flow velocities in patients with proliferative diabetic retinopathy after scatter photocoagulation. Two years of follow-up. Retina 16:222–227
Muqit MM, Gray JC, Marcellino GR, Henson DB, Young LB, Patton N et al (2010) In vivo laser-tissue interactions and healing responses from 20 vs 100-millisecond pulse Pascal photocoagulation burns. Arch Ophthalmol 128:448–455
Paulus YM, Kaur K, Egbert PR, Blumenkranz MS, Moshfeghi DM (2013) Human histopathology of PASCAL laser burns. Eye (Lond) 27:995–996
Ikuno Y, Kawaguchi K, Nouchi T (2010) Choroidal thickness in healthy Japanese subjects. Invest Ophthalmol Vis Sci 51:2173–2176
Michalewski J, Michalewska Z, Nawrocka Z (2014) Correlation of choroidal thickness and volume measurements with axial length and age using swept source optical coherence tomography and optical low-coherence reflectometry. BioMed Res Int 2014:639160
Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jmpel HD, Shah ST et al (1994) Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med 331:1480–1487
Vinores SA, Youssri AI, Luna JD, Chen YS, Bhargavec S, Vinores MA et al (1997) Upregulation of vascular endothelial growth factor in ischemic and nonischemic human and experimental retinal disease. Histol Histopathol 12:99–109
Adamis AP, Miller JW, Bernal MT, D’Amico DJ, Folkman J, Yeo TK et al (1994) Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol 118:445–450
Lains I, Figueira J, Santos AR, Baltar A, Costa M, Nunes S et al (2014) Choroidal thickness in diabetic retinopathy: the influence of antiangiogenic therapy. Retina 34:1199–1207
Sonoda S, Sakamoto T, Yamashita T, Otsuka H, Shirasawa M, Kakiuchi N et al (2014) Effect of intravitreal triamcinolone acetonide or bevacizumab on choroidal thickness in eyes with diabetic macular edema. Invest Ophthalmol Vis Sci 55:3979–3985
Acknowledgements
The authors would like to thank Atsuko Inoue and Mao Sasaki for their help in data collection. The authors would like to thank Enago (www.enago.jp) for the English language review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Ohara, Z., Tabuchi, H., Nakakura, S. et al. Changes in choroidal thickness in patients with diabetic retinopathy. Int Ophthalmol 38, 279–286 (2018). https://doi.org/10.1007/s10792-017-0459-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10792-017-0459-9