Abstract
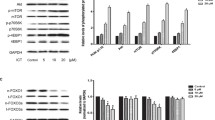
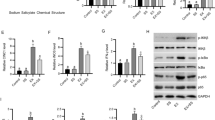
Improving inflammation may serve as useful therapeutic interventions for the hindlimb unloading-induced disuse muscle atrophy. Celecoxib is a selective non-steroidal anti-inflammatory drug. We aimed to determine the role and mechanism of celecoxib in hindlimb unloading-induced disuse muscle atrophy. Celecoxib significantly attenuated the decrease in soleus muscle mass, hindlimb muscle function and the shift from slow- to fast-twitch muscle fibers caused by hindlimb unloading in rats. Importantly, celecoxib inhibited the increased expression of inflammatory factors, macrophage infiltration in damaged soleus muscle. Mechanistically, Celecoxib could significantly reduce oxidative stress and endoplasmic reticulum stress in soleus muscle of unloaded rats. Furthermore, celecoxib inhibited muscle proteolysis by reducing the levels of MAFbx, MuRF1, and autophagy related proteins maybe by inhibiting the activation of pro-inflammatory STAT3 pathway in vivo and in vitro. This study is the first to demonstrate that celecoxib can attenuate disuse muscle atrophy caused by hindlimb unloading via suppressing inflammation, oxidative stress and endoplasmic reticulum stress probably, improving target muscle function and reversing the shift of muscle fiber types by inhibiting STAT3 pathways-mediated inflammatory cascade. This study not only enriches the potential molecular regulatory mechanisms, but also provides new potential therapeutic targets for disuse muscle atrophy.






Similar content being viewed by others
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Chen H, Qian Z, Zhang S, Tang J, Fang L, Jiang F, Ge D, Chang J, Cao J, Yang L et al (2021) Silencing COX-2 blocks PDK1/TRAF4-induced AKT activation to inhibit fibrogenesis during skeletal muscle atrophy. Redox Biol 38:101774
Cheng H, Huang H, Guo Z, Chang Y, Li Z (2021) Role of prostaglandin E2 in tissue repair and regeneration. Theranostics 11:8836–8854
Cui Q, Yang H, Gu Y, Zong C, Chen X, Lin Y, Sun H, Shen Y, Zhu J (2020) RNA sequencing (RNA-seq) analysis of gene expression provides new insights into hindlimb unloading-induced skeletal muscle atrophy. Ann Transl Med 8:1595
Desaphy JF, Pierno S, Liantonio A, Giannuzzi V, Digennaro C, Dinardo MM, Camerino GM, Ricciuti P, Brocca L, Pellegrino MA et al (2010) Antioxidant treatment of hindlimb-unloaded mouse counteracts fiber type transition but not atrophy of disused muscles. Pharmacol Res 61:553–563
Dhapola R, Hota SS, Sarma P, Bhattacharyya A, Medhi B, Reddy DH (2021) Recent advances in molecular pathways and therapeutic implications targeting neuroinflammation for Alzheimer’s disease. Inflammopharmacology 29:1669–1681
Eggelbusch M, Shi A, Broeksma BC, Vazquez-Cruz M, Soares MN, de Wit GMJ, Everts B, Jaspers RT, Wust RCI (2022) The nlrp3 inflammasome contributes to inflammation-induced morphological and metabolic alterations in skeletal muscle. J Cachexia Sarcopenia Muscle 13:3048–3061
Fang WY, Tseng YT, Lee TY, Fu YC, Chang WH, Lo WW, Lin CL, Lo YC (2021) Triptolide prevents LPS-induced skeletal muscle atrophy via inhibiting NF-kappaB/TNF-alpha and regulating protein synthesis/degradation pathway. Br J Pharmacol 178:2998–3016
Farooq F, Abadia-Molina F, MacKenzie D, Hadwen J, Shamim F, O’Reilly S, Holcik M, MacKenzie A (2013) Celecoxib increases SMN and survival in a severe spinal muscular atrophy mouse model via p38 pathway activation. Hum Mol Genet 22:3415–3424
Franco-Romero A, Sandri M (2021) Role of autophagy in muscle disease. Mol Aspects Med 82:101041
Gallot YS, Bohnert KR (2021) Confounding roles of ER stress and the unfolded protein response in skeletal muscle atrophy. Int J Mol Sci 22:2567
Gao S, Zhang G, Zhang Z, Zhu JZ, Li L, Zhou Y, Rodney GG Jr, Abo-Zahrah RS, Anderson L, Garcia JM et al (2022) Ubr2 targets myosin heavy chain IIb and IIx for degradation: molecular mechanism essential for cancer-induced muscle wasting. Proc Natl Acad Sci USA 119:e2200215119
Gonzalez-Jamett A, Vasquez W, Cifuentes-Riveros G, Martinez-Pando R, Saez JC, Cardenas AM (2022) Oxidative stress, inflammation and connexin hemichannels in muscular dystrophies. Biomedicines 10:507
Guadagnin E, Mazala D, Chen YW (2018) Stat3 in skeletal muscle function and disorders. Int J Mol Sci 19:2265
Huang Z, Zhong L, Zhu J, Xu H, Ma W, Zhang L, Shen Y, Law BY, Ding F, Gu X et al (2020) Inhibition of IL-6/JAK/STAT3 pathway rescues denervation-induced skeletal muscle atrophy. Ann Transl Med 8:1681
Huang L, Li M, Deng C, Qiu J, Wang K, Chang M, Zhou S, Gu Y, Shen Y, Wang W et al (2022) Potential therapeutic strategies for skeletal muscle atrophy. Antioxidants 12:44
Ji Y, Li M, Chang M, Liu R, Qiu J, Wang K, Deng C, Shen Y, Zhu J, Wang W et al (2022) Inflammation: roles in skeletal muscle atrophy. Antioxidants 11:1686
Kaneguchi A, Umehara T, Yamaoka K, Ozawa J (2022) Bilateral muscle atrophy after anterior cruciate ligament reconstruction in rats: protective effects of anti-inflammatory drug celecoxib. Knee 35:201–212
Krasselt M, Baerwald C (2019) Celecoxib for the treatment of musculoskeletal arthritis. Expert Opin Pharmacother 20:1689–1702
Lee PHU, Chung M, Ren Z, Mair DB, Kim DH (2022) Factors mediating spaceflight-induced skeletal muscle atrophy. Am J Physiol Cell Physiol 322:C567–C580
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25:402–408
Ma JF, Sanchez BJ, Hall DT, Tremblay AK, Di Marco S, Gallouzi IE (2017) STAT3 promotes IFNgamma/TNFalpha-induced muscle wasting in an NF-kappab-dependent and IL-6-independent manner. EMBO Mol Med 9:622–637
Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, Metzger D, Reggiani C, Schiaffino S, Sandri M (2009) Autophagy is required to maintain muscle mass. Cell Metab 10:507–515
Milan G, Romanello V, Pescatore F, Armani A, Paik JH, Frasson L, Seydel A, Zhao J, Abraham R, Goldberg AL et al (2015) Regulation of autophagy and the ubiquitin-proteasome system by the FoxO transcriptional network during muscle atrophy. Nat Commun 6:6670
Peladeau C, Adam NJ, Jasmin BJ (2018) Celecoxib treatment improves muscle function in MDX mice and increases utrophin A expression. FASEB J 32:5090–5103
Riley JS, Tait SW (2020) Mitochondrial DNA in inflammation and immunity. EMBO Rep 21:e49799
Rom O, Reznick AZ (2016) The role of e3 ubiquitin-ligases MuRF-1 and MAFbx in loss of skeletal muscle mass. Free Radical Biol Med 98:218–230
Romanello V, Sandri M (2010) Mitochondrial biogenesis and fragmentation as regulators of muscle protein degradation. Curr Hypertens Rep 12:433–439
Sartori R, Romanello V, Sandri M (2021) Mechanisms of muscle atrophy and hypertrophy: implications in health and disease. Nat Commun 12:330
Shen Y, Zhang R, Xu L, Wan Q, Zhu J, Gu J, Huang Z, Ma W, Shen M, Ding F et al (2019a) Microarray analysis of gene expression provides new insights into denervation-induced skeletal muscle atrophy. Front Physiol 10:1298
Shen S, Liao Q, Liu J, Pan R, Lee SM, Lin L (2019b) Myricanol rescues dexamethasone-induced muscle dysfunction via a sirtuin 1-dependent mechanism. J Cachexia Sarcopenia Muscle 10:429–444
Shen Y, Li M, Wang K, Qi G, Liu H, Wang W, Ji Y, Chang M, Deng C, Xu F et al (2022) Diabetic muscular atrophy: molecular mechanisms and promising therapies. Front Endocrinol 13:917113
Shimada N, Sakata A, Igarashi T, Takeuchi M, Nishimura S (2020) M1 macrophage infiltration exacerbate muscle/bone atrophy after peripheral nerve injury. BMC Musculoskelet Disord 21:44
Sun J, Yang H, Yang X, Chen X, Xu H, Shen Y, Ding F, Gu X, Zhu J, Sun H (2021) Global alternative splicing landscape of skeletal muscle atrophy induced by hindlimb unloading. Ann Transl Med 9:643
Thoma A, Lightfoot AP (2018) Nf-kb and inflammatory cytokine signalling: role in skeletal muscle atrophy. Adv Exp Med Biol 1088:267–279
Wan Q, Zhang L, Huang Z, Zhang H, Gu J, Xu H, Yang X, Shen Y, Law BY, Zhu J et al (2020) Aspirin alleviates denervation-induced muscle atrophy via regulating the Sirt1/PGC-1α axis and STAT3 signaling. Ann Transl Med 8:1524
Wang W, Shen D, Zhang L, Ji Y, Xu L, Chen Z, Shen Y, Gong L, Zhang Q, Shen M et al (2022) SKP-SC-EVs mitigate denervated muscle atrophy by inhibiting oxidative stress and inflammation and improving microcirculation. Antioxidants 11:66
Wang K, Liu Q, Tang M, Qi G, Qiu C, Huang Y, Yu W, Wang W, Sun H, Ni X et al (2023) Chronic kidney disease-induced muscle atrophy: molecular mechanisms and promising therapies. Biochem Pharmacol 208:115407
Yao C, Narumiya S (2019) Prostaglandin-cytokine crosstalk in chronic inflammation. Br J Pharmacol 176:337–354
Yin L, Li N, Jia W, Wang N, Liang M, Yang X, Du G (2021) Skeletal muscle atrophy: from mechanisms to treatments. Pharmacol Res 172:105807
Yu J, Nagasu H, Murakami T, Hoang H, Broderick L, Hoffman HM, Horng T (2014) Inflammasome activation leads to caspase-1-dependent mitochondrial damage and block of mitophagy. Proc Natl Acad Sci USA 111:15514–15519
Zhang L, Li M, Wang W, Yu W, Liu H, Wang K, Chang M, Deng C, Ji Y, Shen Y et al (2022) Celecoxib alleviates denervation-induced muscle atrophy by suppressing inflammation and oxidative stress and improving microcirculation. Biochem Pharmacol 203:115186
Zhang H, Qi G, Wang K, Yang J, Shen Y, Yang X, Chen X, Yao X, Gu X, Qi L et al (2023) Oxidative stress: roles in skeletal muscle atrophy. Biochem Pharmacol 214:115664
Zimmers TA, Fishel ML, Bonetto A (2016) Stat3 in the systemic inflammation of cancer cachexia. Semin Cell Dev Biol 54:28–41
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (Nos. 92068112, 82072160, 32130060, 81901933), the Major Natural Science Research Projects in Universities of Jiangsu Province (No. 20KJA310012), the Natural Science Foundation of Jiangsu Province (Nos. BK20202013, BK20201209), the “QingLan Project” in Jiangsu Universities, the Priority Academic Program Development of Jiangsu Higher Education Institutions, and Nantong Science and Technology Program (Nos. JC22022037, MS22022010). Nantong university college students’ innovative training program (202310304119Y).
Author information
Authors and Affiliations
Contributions
Conceptualization, X.Y., L.Q. and H.S.; data curation, Y.J., J.L., R.L., K.W. and M.C.; formal analysis, Y.J., R.L., J.L., B.L. and Z.G.; funding acquisition, Y.S. and H.S.; investigation, Y.J., J.L., R.L., K.W. and M.C.; project administration, X.Y. and H.S.; resources, H.S.; supervision, H.S.; writing—original draft, Y.J., J.L., R.L., K.W. and M.C.; writing—review and editing, Y.S., J.Z., X.Y., L.Q. and H.S. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors report that there are no conflicts of interest.
Ethics approval and consent to participate
All animal experiments were performed according to the animal care guidelines of Nantong University and were approved by the Jiangsu Provincial Laboratory Animal Management Committee (No. S20200312-003).
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ji, Y., Lin, J., Liu, R. et al. Celecoxib attenuates hindlimb unloading-induced muscle atrophy via suppressing inflammation, oxidative stress and ER stress by inhibiting STAT3. Inflammopharmacol 32, 1633–1646 (2024). https://doi.org/10.1007/s10787-024-01454-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-024-01454-7