Abstract
Objectives
Considering the prevalence of oral mucositis, we aimed to use the analgesic effects of doxepin with chitosan’s antimicrobial and bio-adhesive nature to fabricate a nano-formulation for treating chemotherapy-induced oral mucositis.
Materials and methods
Nanogel was fabricated via ionic gelation and characterized. Sixty patients were randomly divided and received four different treatments for 14 days: diphenhydramine + aluminum–magnesium mouthwash (control), doxepin mouthwash (DOX MW), chitosan nanogel (CN), and doxepin/chitosan nanogel (CN + DOX). Lesions were assessed with four indices, National Cancer Institute (NCI), World Health Organization (WHO), World Conference on Clinical and Research in Nursing (WCCNR) and visual analog scale (VAS) before and 3, 7, and 14 days after interventions. Kruskal–Wallis test was used for pairwise comparison.
Results
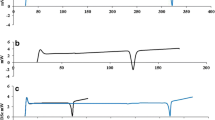
CN had semisolid consistency, uniform spherical shape, an average size of 47.93 ± 21.69 nm, and a zeta potential of + 1.02 ± 0.16 mV. CN + DOX reduced WHO, WCCNR, and VAS scores significantly more than the control three days after the intervention. Seven days after the intervention, CN + DOX reduced NCI and WCCNR considerably more than the control; it reduced WCCNR significantly more than CN. Fourteen days after the intervention, CN + DOX decreased NCI markedly more than the control.
Conclusion
Chitosan-based doxepin nano-formulation might be a promising alternative for routine treatments of oral mucositis.



Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Abdelbary GA, Aburahma MH (2015) Oro-dental mucoadhesive proniosomal gel formulation loaded with lornoxicam for management of dental pain. J Liposome Res 25:107–121
Aduba DC Jr, Hammer JA, Yuan Q, Yeudall WA, Bowlin GL, Yang H (2013) Semi-interpenetrating network (sIPN) gelatin nanofiber scaffolds for oral mucosal drug delivery. Acta Biomater 9:6576–6584
Akbari N, Asadimehr N, Kiani Z (2020) The effects of licorice containing diphenhydramine solution on recurrent aphthous stomatitis: a double-blind, randomized clinical trial. Complement Ther Med 50:102401
Avery, DR, and McDonald, RE. 2010. McDonald and avery dentistry for the child and adolescent-E-book (Elsevier Health Sciences).
Baxter RM, Dai T, Kimball J, Wang E, Hamblin MR, Wiesmann WP, McCarthy SJ, Baker SM (2013) Chitosan dressing promotes healing in third degree burns in mice: gene expression analysis shows biphasic effects for rapid tissue regeneration and decreased fibrotic signaling. J Biomed Mater Res A 101:340–348
Bektas N, Şenel B, Yenilmez E, Özatik O, Arslan R (2020) Evaluation of wound healing effect of chitosan-based gel formulation containing vitexin. Saudi Pharm J 28:87–94
Bey A, Ahmed SS, Hussain B, Devi S, Hashmi SH (2010) Prevention and management of antineoplastic therapy induced oral mucositis. Natl J Maxillofac Surg 1:127–134
Choi JS, Han S-H, Hyun C, Yoo HS (2016) Buccal adhesive nanofibers containing human growth hormone for oral mucositis. J Biomed Mater Res B Appl Biomater 104:1396–1406
Chou CI, Shih CJ, Chen YT, Ou SM, Yang CY, Kuo SC, Chu D (2016) Adverse effects of oral nonselective and cyclooxygenase-2-selective NSAIDs on hospitalization for acute kidney injury: a Nested Case-Control Cohort Study. Medicine (baltimore) 95:e2645
Choudhary A, Kant V, Jangir BL, Joshi VG (2020) Quercetin loaded chitosan tripolyphosphate nanoparticles accelerated cutaneous wound healing in Wistar rats. Eur J Pharmacol 880:173172
Conklin KA (2004) Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr Cancer Ther 3:294–300
Dhawan N, KrishanKaliaSaahil KANA (2014) N-succinyl chitosan as buccal penetration enhancer for delivery of herbal agents in treatment of oral mucositis. Curr Drug Deliv 11:415–425
Eivazzadeh-Keihan R, Khalili F, Aliabadi HAM, Maleki A, Madanchi H, Ziabari EZ, Bani MS (2020) Alginate hydrogel-polyvinyl alcohol/silk fibroin/magnesium hydroxide nanorods: a novel scaffold with biological and antibacterial activity and improved mechanical properties. Int J Biol Macromol 162:1959–1971
El-Shabouri MH, Abd El-Aleem M, Hamdy Soliman OA, El-Dahhan MS (2009) Formulation and evaluation of a buccoadhesive captopril tablets. Bull Pharm Sci Assiut 32:45–64
Epstein JB, Truelove EL, Oien H, Allison C, Le ND, Epstein MS (2001) Oral topical doxepin rinse: analgesic effect in patients with oral mucosal pain due to cancer or cancer therapy. Oral Oncol 37:632–637
Epstein JB, Epstein JD, Epstein MS, Oien H, Truelove EL (2006) Oral doxepin rinse: the analgesic effect and duration of pain reduction in patients with oral mucositis due to cancer therapy. Anesth Analg 103:465–470
Epstein JB, Epstein JD, Epstein MS, Oien H, Truelove EL (2008) Doxepin rinse for management of mucositis pain in patients with cancer: one week follow-up of topical therapy. Spec Care Dentist 28:73–77
Gilhotra RM, Ikram M, Srivastava S, Gilhotra N (2014) A clinical perspective on mucoadhesive buccal drug delivery systems. J Biomed Res 28:81–97
Giunchedi P, Juliano C, Gavini E, Cossu M, Sorrenti M (2002) Formulation and in vivo evaluation of chlorhexidine buccal tablets prepared using drug-loaded chitosan microspheres. Eur J Pharm Biopharm 53:233–239
Goy RC, de Britto D, Assis OBG (2009) A review of the antimicrobial activity of chitosan. Polímeros 19:241–247
Howling GI, Dettmar PW, Goddard PA, Hampson FC, Dornish M, Wood EJ (2001) The effect of chitin and chitosan on the proliferation of human skin fibroblasts and keratinocytes in vitro. Biomaterials 22:2959–2966
Huebner KL, Kunkel AK, McConnel CS, Callan RJ, Dinsmore RP, Caixeta LS (2017) Evaluation of horn bud wound healing following cautery disbudding of preweaned dairy calves treated with aluminum-based aerosol bandage. J Dairy Sci 100:3922–3929
Ikeuchi-Takahashi Y, Sasatsu M, Onishi H (2013) Evaluation of matrix type mucoadhesive tablets containing indomethacin for buccal application. Int J Pharm 453:454–461
Jafari A, Hassanajili S, Karimi MB, Emami A, Ghaffari F, Azarpira N (2018) Effect of organic/inorganic nanoparticles on performance of polyurethane nanocomposites for potential wound dressing applications. J Mech Behav Biomed Mater 88:395–405
Jonathan AE, Desotelle JA, Wilding G, Jarrard DF (2010) Therapy-induced senescence in cancer. JNCI 102:1536–46
Kong M, Chen XG, Xing Ke, Park HJ (2010) Antimicrobial properties of chitosan and mode of action: a state of the art review. Int J Food Microbiol 144:51–63
Kravanja G, Primožič M, Knez Ž, Leitgeb M (2019) Chitosan-based (Nano) materials for novel biomedical applications. Molecules 24:1960
Kusiak A, Jereczek-Fossa BA, Cichońska D, Alterio D (2020) Oncological-therapy related oral mucositis as an interdisciplinary problem—literature review. Int J Environ Res Public Health 17:2464
Kwon Y (2016) Mechanism-based management for mucositis: option for treating side effects without compromising the efficacy of cancer therapy. Onco Targets Ther 9:2007
Lopez TC, Martins MD, Pavesi VC, Ferreira LS, Bussadori SK, Moreira MS, Marques MM (2013) Effect of laser phototherapy in the prevention and treatment of chemo-induced mucositis in hamsters. Braz Oral Res 27:342–348
Lozano DD, Noordenbos J, Hansbrough JF (2002) The use of Glucan II in the treatment of donor sites. J Burn Care Rehabil 23:S81
Makvandi P, Ali GW, Sala FD, Abdel-Fattah WI, Borzacchiello A (2019) Biosynthesis and characterization of antibacterial thermosensitive hydrogels based on corn silk extract, hyaluronic acid and nanosilver for potential wound healing. Carbohyd Polym 223:115023
Meabed OM, Shamaa A, Abdelrahman IY, El-Sayyed GS, Mohammed SS (2022) The effect of Nano-chitosan and Nano-curcumin on radiated parotid glands of Albino rats: comparative study. J Cluster Sci 34:977–989
Mohammed E, Aboulkhair AG, Tawifq MM (2022) Effect of nano-chitosan and nano-doxycycline gel on healing of induced oral ulcer in rat model: histological and immunohistochemical study. Clin Oral Invest 26:3109–3118
Oien, Hal, Edmond Truelove, and Joel Epstein (2005) Compositions and methods of administering doxepin to mucosal tissue. In.: Google Patents
Park CJ, Clark SG, Lichtensteiger CA, Jamison RD, Johnson AJ (2009) Accelerated wound closure of pressure ulcers in aged mice by chitosan scaffolds with and without bFGF. Acta Biomater 5:1926–1936
Patrulea V, Ostafe V, Borchard G, Jordan O (2015) Chitosan as a starting material for wound healing applications. Eur J Pharm Biopharm 97:417–426
Potrč T, Baumgartner S, Roškar R, Planinšek O, Lavrič Z, Kristl J, Kocbek P (2015) Electrospun polycaprolactone nanofibers as a potential oromucosal delivery system for poorly water-soluble drugs. Eur J Pharm Sci 75:101–113
Reda RI, Wen MM, El-Kamel AH (2017) Ketoprofen-loaded Eudragit electrospun nanofibers for the treatment of oral mucositis. Int J Nanomedicine 12:2335
SaberzadehZabihi EM, Motaghi E, Shishehbor Fatemeh (2021) Doxepin accelerates the healing process of burn wounds in mice. Thai J Pharma Sci 45:100–104
Sanz R, Calpena AC, Mallandrich M, Gimeno Á, Halbaut L, Clares B (2017) Development of a buccal doxepin platform for pain in oral mucositis derived from head and neck cancer treatment. Eur J Pharm Biopharm 117:203–211
Sezer AD, Cevher E (2012) Topical drug delivery using chitosan nano-and microparticles. Expert Opin Drug Deliv 9:1129–1146
Sio TT, Le-Rademacher JG, Leenstra JL, Loprinzi CL, Rine G, Curtis A, Singh AK, Martenson JA Jr, Novotny PJ, Tan AD, Qin R, Ko SJ, Reiter PL, Miller RC (2019) Effect of Doxepin mouthwash or diphenhydramine-lidocaine-antacid mouthwash vs placebo on radiotherapy-related oral mucositis pain: the alliance A221304 randomized clinical trial. JAMA 321:1481–1490
Sonis ST (2009) Mucositis: the impact, biology and therapeutic opportunities of oral mucositis. Oral Oncol 45:1015–1020
Srinivas M, Medaiah S, Girish S, Anil M, Pai J, Walvekar A (2011) The effect of ketoprofen in chronic periodontitis: a clinical double-blind study. J Indian Soc Periodontol 15:255–259
Tungprapa S, Jangchud I, Supaphol P (2007) Release characteristics of four model drugs from drug-loaded electrospun cellulose acetate fiber mats. Polymer 48:5030–5041
Valerie K, Yacoub A, Hagan MP, Curiel DT, Fisher PB, Grant S, Dent P (2007) Radiation-induced cell signaling: inside-out and outside-in. Mol Cancer Ther 6:789–801
Wang T, Zheng Y, Shen Y, Shi Y, Li F, Chang Su, Zhao L (2018) Chitosan nanoparticles loaded hydrogels promote skin wound healing through the modulation of reactive oxygen species. Artif Cell Nanomed Biotechnol 46:138–149
Yedurkar P, Dhiman MK, Petkar K, Sawant K (2012) Mucoadhesive bilayer buccal tablet of carvedilol-loaded chitosan microspheres: in vitro, pharmacokinetic and pharmacodynamic investigations. J Microencapsul 29:126–137
Zheng W, Hao Y, Wang D, Huang H, Guo F, Sun Z, Shen P, Sui K, Yuan C, Zhou Q (2021) Preparation of triamcinolone acetonide-loaded chitosan/fucoidan hydrogel and its potential application as an oral mucosa patch. Carbohyd Polym 272:118493
Zhou Y, Yao H, Wang J, Wang D, Liu Q, Li Z (2015) Greener synthesis of electrospun collagen/hydroxyapatite composite fibers with an excellent microstructure for bone tissue engineering. Int J Nanomedicine 10:3203–3215
Zu G, Steinmüller M, Keskin D, van der Mei HC, Mergel O, van Rijn P (2020) Antimicrobial nanogels with nanoinjection capabilities for delivery of the hydrophobic antibacterial agent triclosan. ACS Appl Polym Mater 2:5779–5789
Acknowledgements
With this, we gratefully acknowledge the participants in this study, the vice chancellor for research of Shiraz University of Medical Sciences, Daroo Pakhsh Co., for providing us with doxepin powder, and Dr. Vosough, who helped us with the statistical analysis of this project.
Funding
This study was funded by the vice chancellor for research of Shiraz University of Medical Sciences.
Author information
Authors and Affiliations
Contributions
M.R. and M.M. contributed to the conception, study design, and critically revised the manuscript. A.G. contributed to the conception, study design, formulation preparation, and critically revised the manuscript. N.S. contributed to data acquisition and interpretation, and wrote the main manuscript, and A.A. contributed to study design and data acquisition. All authors were aware of all parts of the study, gave their final approval, and agreed to be held accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no competing interests, personal or financial relationships that could have affected the results of this study.
Ethical approval
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki, and the Ethical Committees of Shiraz University of Medical Sciences approved the study by ethical code IR.SUMS.DENTAL.REC.1400.085 and clinical trial code IRCT20110428006322N4. Written consent was taken from participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Samiraninezhad, N., Rezaee, M., Gholami, A. et al. A novel chitosan-based doxepin nano-formulation for chemotherapy-induced oral mucositis: a randomized, double-blinded, placebo-controlled clinical trial. Inflammopharmacol 31, 2411–2420 (2023). https://doi.org/10.1007/s10787-023-01325-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10787-023-01325-7