Abstract
The use of supercritical fluids in technical applications requires an accurate knowledge of their critical points. For mixtures, these can deviate significantly and without a linear dependency from the critical points of its individual pure components. Since even small amounts of admixture can have noticeable effects, this not only concerns blends of targeted compositions, but also unintentional mixtures for example caused by impurities. Within this work, a method for the calculation of critical points is presented which focuses on numerical robustness promoting a fast and reliable generation of results. Implemented into the thermodynamic property software TREND, with its mixture modeling capabilities, the method allows a flexible combination of different equations of state and mixture models which also includes predictive approaches. Against the background of an increasing relevance of mixtures based on supercritical CO\(_2\) (sCO\(_2\)) for energy applications, critical lines are calculated and compared against experimental results for selected sCO\(_2\)-based mixtures recently considered for power plant applications. Herein, several combinations of equations of state (EoS) and mixture models are compared. Critical lines are calculated for the first time in this work with the combination of the multi-fluid mixture model with excess Gibbs energy (\(g^\text {E}\)) models. It was found that the critical lines calculated with the combination of the multi-fluid mixture model with the \(g^\text {E}\)-model COSMO-SAC yields good predictive results for the investigated CO\(_2\) mixtures.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
1 Introduction
The special properties of supercritical fluids and their mixtures open up a wide range of possibilities for various technical applications. In this context, due to an easy availability at low cost, non-toxicity and outstanding solvent properties, CO\(_2\) in particular plays an important role in several industrial applications such as extraction and purification processes or supercritical drying [1]. Moreover, supercritical CO\(_2\) (sCO\(_2\)) gets an increasing attraction in the field of energy technology. Its favorable location of the critical point, close to typical ambient temperatures, combined with its high temperature stability allows a wide applicability to different energy sources of conventional and regenerative origin. The near critical properties like, e.g., high densities and low viscosities promise significant increases in efficiency while potentially reducing the complexity and size of individual components. This enables the reasonable and more complete use of available energy sources which are not or only partially usable with existing technologies. If, in addition, supercritical mixtures are used instead of pure CO\(_2\), further improvements may be possible for example by better adapting the location of the critical point to pre-given boundary conditions or by improving size and efficiency of key components (see e.g. [2,3,4,5]).
However, as even small amounts of admixture may have significant influence on its location [3], across all applications, the reliable prediction of the critical point of mixtures not only concerns blends of targeted compositions, but also unintentional mixtures for example caused by impurities. Moreover, it was already shown, that the involved equations of state and mixture models may have significant influence on the critical lines as well as on the overall results for technical applications in this region [3, 6,7,8,9]. At the same time, more and more mixtures are being considered for sCO\(_2\) power plant applications, for which no adjusted or only simple models exist (e.g. CO\(_2\)+Xe, CO\(_2\)+TiCl\(_4\), CO\(_2\)+SF\(_6\), cf. [3, 10,11,12]). Accordingly, calculation methods are required which, for a selected blend, allow to combine the best available models including predictive approaches and which can be applied easily within technical applications.
Based on the constraints on defining a critical phase stated by Gibbs in 1878 [13], a number of calculation procedures has been established over time. For instance, as an early work, Redlich and Kister [14] derived a basic calculation approach for binary mixtures based on the fugacity coefficients. Spear et al. [15, 16] calculated vapor-liquid critical states for binary and ternary mixtures by searching extremum pressures on the fluids instability curves using the Redlich-Kwong equation of state (EoS). Van Konynenburg and Scott [17] used the van der Waals equation to calculate critical lines and phase diagrams. Baker and Luks [18] presented an iterative method to solve the critical criteria using a modified version of the Redlich-Kwong EoS including methods to find appropriate starting values. As a frequently cited work, Heidemann and Khalil [19] reformulated the stability criteria in terms of an expansion of the Helmholtz free energy in a Taylor series. This leads to a sum-term formulation which significantly reduces the computational effort compared to earlier methods. By using the Soave-Redlich-Kwong (SRK) EoS, critical properties were calculated for selected examples of binary and higher order mixtures. Subsequently, this method was taken up by several authors. Michelsen and Heidemann [20] modified the original method to further reduce the computing time. Kolár and Kojima [21] extended the Heidemann and Khalil approach by the predictive Soave-Redlich-Kwong (PSRK) model [22, 23] including its analytical derivatives to predictively calculate multicomponent critical points for a total of 36 mixtures. Stockfleth and Dohrn [24] used the method by Heidemann and Khalil [19] including modifications by Michelsen [20] to compile an algorithm independent of specific equations of state and mixture models by relying on a numerical calculation of the derivatives. Example calculations were done for selected binary and ternary mixtures by an implementation of their algorithm in the commercial software tool ASPEN PLUS using the Peng-Robinson EoS and the SRK EoS combined with the PSRK model. Bell and Jäger [25] developed a method for calculating all critical points of mixtures using analytic derivatives of Helmholtz-energy-explicit mixture models. They found that multiparameter equations of state tend to have false solutions for the critical point criterion which complicates the evaluation of critical points for this type of EoS. Deiters and Bell [26] presented a robust method on calculating critical points by numerically integrating sets of differential equations. Critical lines for a selection of binary and ternary mixtures were calculated with different model combinations including the GERG 2008 model(s) [27] and the Peng-Robinson EoS. Applied to a selection of binary mixtures of hydrocarbons in a subsequent work [28], they could show unphysical behavior of several model combinations as also mentioned in the work of Bell and Jäger [25]. This in turn emphasizes the need for a reliable implementation of a critical point algorithm, allowing the best possible freedom in the selection and combination of different EoS and mixture models.
Related to this, this paper presents the implementation of a critical point algorithm in the thermodynamic property software TREND [29], which has been developed under the lead of Prof. Roland Span at Ruhr-University Bochum, and its application to a selection of CO\(_2\) mixtures recently considered for power plant applications. Herein, emphasis was placed on its flexible usability with the EoS and mixture models available in TREND, and particularly with regard to the applicability of higher-order predictive approaches. Based on the method of Heidemann and Khalil [19], as well as on the additions of Michelsen [30] and Stockfleth and Dohrn [24], a completely numerical approach was chosen and extended in terms of numerical stability. Applied to a selection of CO\(_2\) mixtures, the algorithm was validated for different mixture models and equations of state. In addition, the calculated critical points of several EoS and mixture models were compared to each other. Critical lines calculated with the combination of the multi-fluid mixture model and the excess Gibbs energy (\(g^\text {E}\)) model COSMO-SAC are published for the first time in this article.
1.1 Fluid Modeling
Due to the full integration of the algorithm into the thermodynamic property software TREND [29] and the use of numerical derivatives, all EoS and mixture models which are available in the software can be used for calculating critical lines. Out of these, in this work, multiparameter equations of state as well as the Soave-Redlich-Kwong (SRK) equation of state were used in combination with the multi-fluid mixture model, standard SRK mixing rules and the excess Gibbs energy (\(g^\text{E}\)) model COSMO-SAC. With reference to this, these models are briefly introduced in the following section.
Multiparameter equations of state formulated in the dimensionless Helmholtz energy \(\alpha\) are currently the most advanced method for accurate modeling of thermodynamic properties [31]. On the basis of empirically obtained parameters fitted to a preferably huge database of experimental values, they intend to represent all experimental data within the measurement uncertainty. The dimensionless Helmholtz energy \(\alpha\) is usually composed of an ideal gas term \(\alpha ^0\) and a residual term \(\alpha ^\text {r}\) which accounts for the deviation from the ideal fluid model:
Herein, \(\tau\) and \(\delta\) are, respectively, the reciprocal reduced temperature and reduced density,
in which T denotes the temperature, \(\rho\) the molar density and, indicated by the subscript c, their respective values at the critical point. While \(\alpha ^0\) can be obtained from experimental or simulated data of the isobaric heat capacity (or isochoric heat capacity or speed of sound data) in combination with the ideal gas law, \(\alpha ^\text {r}\) consists of different sets of empirical terms (polynomial terms, exponential terms, Gaussian-bell shaped terms, and non-analytical terms), which are fitted to experimental data.
Whereas for the multiparameter equations of state a sufficient experimental basis must be available, cubic equations of state, including the SRK, typically only require the knowledge of the critical quantities (i.e. \(T_\text {c}\), \(\rho _\text {c}\)) and the acentric factor \(\omega\). Within TREND, the SRK EoS is included on the basis of the residual dimensionless Helmholtz energy \(\alpha ^\text {r}\), which, as described by Bell and Jäger [32], reads:
where a and b are constants to account for the attractive interaction between the molecules and the volume occupied by the molecules, respectively. A more detailed description can be found in the work of Bell and Jäger [32].
Besides equations of state to represent the pure fluid properties, adequate mixture models are required. Analogously to the multiparameter EoS for pure fluids, the multi-fluid mixture model can be considered as the actual state of the art to model the behavior of mixtures [27, 33]. Based on the works of Lemmon and Tillner-Roth [34], Kunz et al. [27, 33], Gernert and Span [35] and Herrig [36], its implementation in TREND has the form
where \(\alpha\) is the dimensionless Helmholtz energy, \(\varvec{\textbf{x}}\) the composition vector of the mixture with \(x_i\) denoting the molar fraction of the component i, and N is the total number of components of the mixture. \(\alpha ^0_{\text {o}i}\) describes the ideal gas contribution for each component, depending on the temperature T and the molar density \(\rho\). Similarly, \(\alpha ^\text {r}_{\text {o}i}\) denotes the residual part of the dimensionless Helmholtz energy of the component i, which can be gained by any equation of state formulated in terms of the residual dimensionless Helmholtz energy, such as the aforementioned multiparameter EoS or, i.e., referring to Eq. 3, simpler formulations such as cubic EoS, see, e.g., [7]. Furthermore, \(\alpha _{ij}^\text {r}\) is the binary specific departure function of the component-tuple ij with \(F_{ij}\) as a parameter and, as before, \(\tau\) and \(\delta\) represent reduced temperature and reduced density:
However, unlike the equations of state for pure fluids (cf. Equation 2), the reducing temperature \(T_\text {r}\) and the reducing density \(\rho _\text {r}\) do not directly correspond to the critical properties but are formulated as a function of the composition of the mixture, i.e., according to Klimeck [37] and Kunz et al. [27, 33]:
and
\(\beta _{T,ij}\), \(\gamma _{T,ij}\), \(\beta _{v,ij}\) and \(\gamma _{v,ij}\) are adjustable parameters, which can be fitted to experimental data for the respective binary mixture of components i and j.
Referring to the cubic equations of state and in particular to the SRK, the standard representation of mixtures is carried out by applying mixing rules to the parameters a and b. As described by Bell and Jäger [32], a is modeled by a quadratic mixing rule:
with
where \(k_{ij}\) is a binary interaction parameter which has to be fitted to experimental data or alternatively may be set to zero for predictive use. b is modeled by a simple linear mixing rule only based on the molar fraction \(x_i\) of each component:
Equivalently to setting \(k_{ij}\) to zero for the SRK, also the multi-fluid mixture model may be used predictively, if no adjusted parameters are available.
At its most basic level, \(T_\text {r}\) and \(\rho _\text {r}\), needed for the multi-fluid mixture model, can be evaluated either by linear mixing rules:
or by Lorentz-Berthelot combining rules by setting \(\beta _{T,ij}\), \(\gamma _{T,ij}\), \(\beta _{v,ij}\) and \(\gamma _{v,ij}\) in Eqs. 6 and 7 to unity each. Note that the departure function \(\alpha _{ij}^\text {r}(\tau ,\delta )\) in Eq. 4 is omitted in both cases. However, as for example shown by Jäger et al. [38, 39] or Rath et al. [3], linear mixing rules or Lorentz-Berthelot combining rules typically don’t yield good results. Connected with this, and to increase the predictive capabilities of the multi-fluid mixture model, Jäger et al. [38, 39] proposed a combination with excess Gibbs energy (\(g^\text {E}\)) models, which results in a predictively applicable, theoretically based departure function. Referring to the original formulation of the multi-fluid mixture model shown in Eq. 4, this results in
where the second term has been replaced by the theoretically based formulation of the departure function, in which \(g_{\text {GE}}^{\text {E,r}}\) is the residual part of the excess Gibbs energy, which can be calculated by arbitrary excess Gibbs energy models such as COSMO-SAC or UNIFAC. Furthermore, b denotes the co-volume of the mixture, \(\rho _\text {ref}\) the density of the mixture at the reference pressure of \(p_0=101325\) Pa, given as a linearly mixed composition of the respective reference density of each component. The reduced properties are given by \(\delta _{\text {ref}}=\rho _\text {ref}/\rho _\text {r},\) \(\delta _{\text {ref},i}=\rho _{\text {ref},i}/\rho _{\text {c},i}\), and \(\tau _i=T_{\text {c},i}/T\). A more detailed description of the model can be found in the works of Jäger et al. [38, 39].
Within this work, the COSMO-SAC model and its extended COSMO-SAC-dsp version, both by Hsieh et al. [40, 41] were used to calculate the residual part of the excess Gibbs energy \(g_{\text {GE}}^{\text {E,r}}\). Both models are improved versions of the original formulation by Lin and Sandler [42, 43], which is based on the conductor-like screening model (COSMO) and the conductor-like screening model for real solvents (COSMO-RS) developed by Klamt and coworkers [44, 45]. Herein, the basic idea is to calculate surface screening charges \(\sigma\) of the molecules, which are then averaged over a finite amount of standard surface elements m, resulting in a set of standard surface charges \(\sigma _m\). The probability \(p_i(\sigma _m)\) of finding a surface with a defined charge \(\sigma _m\) on a molecule i is then described by taking into account the bonding influences of the molecule. Hsieh et al. [40] used a split-up profile in their model by considering surface elements that are non-hydrogen-bonding groups (nhb), hydrogen-bonding hydroxyl groups (OH), and hydrogen-bondings due to other relatively strongly charged atoms (OT) for each molecule:
The sigma-profiles are an input to the COSMO-SAC model and therefore sigma-profiles calculated from COSMO-calculations need to be available to apply the model. In this work, the sigma-profiles provided in the open-source implementation of COSMO-SAC by Bell et al. [46] have been used. In a mixture, the mixed sigma-profile can be calculated from the sigma-profiles of the components that the mixture consists of in the following way:
On this basis, segment activity coefficients \(\Gamma _i^t\) and \(\Gamma _{\text {S}}^t\) are calculated, for which the sigma-profiles of the pure components \(p_i(\sigma )\) and the sigma-profiles of the mixture \(p_{\text {S}}(\sigma )\) are required, respectively. Note that the superscript t stands for either nhb, OH, or OT. The residual activity coefficients \(\gamma ^\text {r}_{i,\text {S}}\) for each component i in the mixture can then be obtained according to:
Finally, the residual part of the excess Gibbs energy \(g_{\text {GE}}^{\text {E,r}}\) results from a summation of the molar fraction weighted natural logarithm of residual activity coefficients \(\gamma ^\text {r}_\text {S}\) for each component:
A more detailed description of the model can be found in the works of Lin and Sandler [42, 43] and Hsieh et al. [40, 41] as well as in the work by Klamt und Schüürmann [44] for the calculation of the sigma profiles.
Hsieh et al. [41] extended the model of Hsieh et al. [40] by additionally considering the dispersive interactions between the molecules using a dispersion parameter \(\varepsilon\), which is used to calculate a parameter A with which activity coefficients can be calculated using a simple one-constant Margules approach for a binary mixture consisting of components 1 and 2
The residual part of the excess Gibbs energy can then be obtained as follows
For more details regarding the dsp extension of COSMO-SAC, the interested reader is referred to the article of Hsieh et al. [41].
2 Model Formulation
2.1 Method of Heidemann and Khalil
Heidemann and Khalil [19] reformulated the Gibbs definition of stability criteria by a Taylor series for the Helmholtz free energy A, resulting in a combination of two summation terms:
Herein, the stability of multiple coexisting phases is given for all positive results for any changes in the mole number \(\Delta n\) of each component compared to the initial state denoted by the subscript 0. A critical point is found for the stability limit at any resulting zero value of the whole expression. As this leads to a positive-semidefiniteness of the quadratic form, implying its determinant to be greater or equal than zero, each of the two summation terms in Eq. 20 must equate to zero. For the first part, i.e., the double sum, this leads to the following equivalent conditions:
where \({\varvec{Q}}\) is a matrix defined by:
and \(\Delta {\varvec{n}}\) is a unique vector (except its scalar multiples), which is represented by the differences in the mole numbers for each component:
As a second part, for an existing vector \(\Delta {\varvec{n}}\), the second summation term of Eq. 20 must be zero. In order to avoid higher order partial derivatives, referring to the work of Michelsen [30], the triple sum term can be reformulated to:
In which the resulting second matrix \(\varvec{Q^*}\) is a partial composition derivative of \({\varvec{Q}}\) with its entries:
and s denoting a small factor to get an infinitesimally small change in the composition. Consequently, complementing the conditions of the double sum in Eq. 21, the third condition for a critical point results from Eq. 24 in:
2.2 Numerical Derivation of the Matrix Elements
By using thermodynamic identities, as also mentioned by Heidemann and Khalil [19], the second derivative of the Helmholtz free energy by the mole numbers from Eq. 22 can be expressed in terms of fugacity:
As the multiplication of a matrix by scalars (here RT) does neither influence the root of its determinant \((\text {det}\left( {\varvec{Q}}\right) =0)\) nor the solution of the system of linear equations, i.e., given by the second condition in Eq. 21, the elements of \({\varvec{Q}}\) from Eq. 22 can be reduced to:
Since now only one partial derivation is necessary, and following the work of Stockfleth and Dohrn [24], this can readily be done numerically using finite differentiation schemes with a finite increment h, e.g., in one of its simplest formulations, in terms of a first order forward differentiation step:
in which \(\varvec{\tilde{n}}\) is the normalized vector of mole numbers of the individual components, with the component \(n_j\) being increased by the k-multiple of the increment h. Adequately, sticking to the example of a first order forward differentiation step, also Eq. 25 can be expressed by finite differentiation depending on the entries of \({\varvec{Q}}\):
Herein, the double tilde notations refer to a simultaneous variation of all mole numbers by a k-multiple of a small change in the mole number variation \(\Delta {\varvec{n}}\) and the respective adaption in the specific volume (\(\tilde{\tilde{v}}\)) referring to the requirement of a constant total volume V.
Both differentiations can be extended to higher-order differentiation schemes for a better accuracy on cost of an increase in the computational effort. For example, Stockfleth and Dohrn [24] propose a five-point central differentiation step scheme while, in the context of this work, central differentiation step schemes up to seven points were used. Apart from that, a significant reduction in computation time can be achieved by taking advantage of the symmetry of matrices \({\varvec{Q}}\) and \(\varvec{Q^*}\). As this implies that \(q_{ij}\hat{=}q_{ji}\) and \(q^*_{ij}\hat{=}q^*_{ji}\), it is only necessary to calculate either the upper or lower triangular matrix, which can then be mirrored across its diagonal.
2.3 Numerical Calculation Procedure
Figure 1 shows a flowchart of the algorithm and how it’s connected to the standard TREND routines. Following the procedure of Heidemann and Khalil [19], the calculation is carried out in two nested iteration loops. Therein, starting from adequate initial values, both terms of the sum are evaluated iteratively until all critical conditions are fulfilled within preset error tolerances. As the reliable convergence of the method depends particularly on the choice of appropriate starting values (cf. e.g. [19, 24, 30]), in a first step, \(T_{\textrm{c,guess}}\) and \(v_{\textrm{c,guess}}\) of the mixture are estimated if they were not explicitly given within the model definition when calling the routine. Herein, the critical temperature is estimated as proposed by Li [47] as the critical volumetric average of the critical temperatures of its components.
Please note that the sum sign in the denominator in the formula for \(\phi _{\textrm{c}i}\) is missing in the original source. The critical molar volume is simply estimated by linear mixing based on the mass-fractions of the individual components:
Furthermore, it can be noted that Heidemann and Khalil [19] recommended setting the initial guess for \(T_\textrm{c}\) to a sufficiently (too) high value by additionally multiplying it by 1.5. Related to this, the parameter \(f_T\) is provided within the implementation, allowing to multiply the temperature guess at each call of the inner routine by a freely configurable factor:
However, within the calculations carried out in this work, the estimated values for \(T_\textrm{c}\) by Eq. 32 were mostly sufficient without an additional multiplication by \(f_T\). Additionally, a similar factor \(f_v\) is provided, equivalently allowing to alter the initial volume guess within the first call of the outer iteration loop.
Starting with an adequate initial value \(T_\textrm{c, guess,0}\), and following the flowchart in Fig. 1, the first sum term is evaluated recurrently by iteratively correcting \(T_\text {c}\) until the first critical condition (cf. Eq. 21) is met, i.e., until the determinant of \({\varvec{Q}}\) equals to zero within a preset error tolerance. While past studies mostly rely on Newton’s method (1st order) for the correction of \(T_\textrm{c}\) (and \(v_\textrm{c}\)), within this work, 2nd and 3rd order Householder methods were used:
in which \(f(T_\text {c})_i=\text {det}\left( {\varvec{Q}}\right) _i\) for the actual iteration i, while \(f'\), \(f''\) and \(f'''\) are the respective first, second, and third order derivatives. Analogous to the derivation of the matrix elements \(q_{ij}\), these were evaluated numerically using central differentiation step schemes up to a five-point stencil. In most cases of the model configurations studied here, the increased computational effort resulting from the need for second and third order derivations was compensated by a significantly better convergence and thus fewer iterations. Furthermore, especially for the \(g^\text {E}\) based mixture models, this resulted in a remarkable increase in stability as higher order methods tend to overestimate less in early iterations. Additionally, a relaxation factor \(\psi _T\) is used which limits the rate of change in the values for \(T_\text {c}\) which also leads to an increased stability, especially when the guess value is relatively far away from the iteration result. In this way, the value for the next iteration step can be obtained as follows:
In order not to slow down the convergence too much, the factor is dynamically adjusted via the actual error and the targeted error tolerance:
Thus, on the one hand, the factor is bounded downwards toward a preset value (i.e., 0.50) and on the other hand, with a decreasing value for the error, it approaches 1, i.e., resulting in an unrelaxated iteration. It should be noted that the value of 0.50 used as the lower limit to bound the relaxation is rather conservative. This value can be set freely as an input parameter in the implemented routines if desired, e.g. to a higher value for faster convergence.
After a suitable value for \(T_\text {c}\) is found in the inner loop, a vector \(\Delta {\varvec{n}}\) is calculated by searching a non-trivial solution for the system of linear equations given by the second critical condition in Eq. 21. Subsequently, continuing the outer loop, \(\varvec{Q^*}\) is compiled and used in combination with \(\Delta {\varvec{n}}\) to evaluate the third critical condition given by Eq. 26. If the resulting value is not within the set error tolerance, \(v_\text {c}\) is adjusted analogously to the iterative correction of \(T_\text {c}\) described by Eqs. 35–38 and by replacing \(a(T_\text {c})_n\) by \(a(v_\text {c})_n=\Delta {\varvec{n}}^\text {T} \varvec{Q^*} \Delta {\varvec{n}}\) (cf. Equation 26). The new value is then used for a new run including the inner loop until a resulting combination of values for \(T_\text {c}\) and \(v_\text {c}\) is found by which all three critical conditions are satisfied. Finally, the critical pressure \(p_\text {c}\) and the critical density \(\rho _\text {c}\) are evaluated using the selected combination of EoS and mixture model.
As can be seen in the flowchart in Fig. 1, the algorithm makes use of several standard routines of the software TREND at a total of three positions. When composing the matrices \({\varvec{Q}}\) and \(\varvec{Q^*}\) within the iteration loops, fugacities are calculated by
where \(\phi _i\) denotes the fugacity coefficient provided by the routines FUGCOPURE_CALC and FUGCO_CALC_MIX and p is the pressure calculated by calling P_calc. Furthermore, in the last step, P_calc is used to calculate the critical pressure from the resulting values for \(T_\text {c}\) and \(v_\text {c}\).
For the calculation of entire critical lines (of type I mixtures according to the classification of mixtures given by Scott and van Konynenburg [48]) calculations can be started at the critical point of a pure component tracing the entire critical line to the critical point of the other component. A suitably small step size needs to be set and the calculation results (or the pure component values) of the last iteration step can be used as the initial guess for the next point on the line.
The implementation of the algorithm in TREND can be used in two core routines for the calculation of single critical points (CRIT_PROPS) or complete critical lines, resp. areas/data sets for mixtures of higher order (CRIT_LINE). A description of the interfaces, including sample calls, is available in the supplementary material.
3 Calculation of Critical Lines for Selected Mixtures
To validate the functionality of the routine and for comparison of the different fluid models, the routine was applied to a selection of binary CO\(_2\)-mixtures recently considered as additives for sCO\(_2\) power cycles. As a first group, the alkanes up to hexane were selected. For example Conboy et al. [12] considered the admixture of n-butane (C\(_4\)H\(_{10}\)) to sCO\(_2\) for geothermal applications. Valencia-Chapi et al. [5] investigated the blending of various hydrocarbons, including propane (C\(_3\)H\(_{8}\)), n-butane (C\(_4\)H\(_{10}\)), and n-pentane (C\(_5\)H\(_{12}\)), with CO\(_2\) for concentrated solar power (CSP) applications. Furthermore, within a systematic screening of potential additives to improve sCO\(_2\) cycles for waste heat recovery by Rath et al. [3], propane (C\(_3\)H\(_{8}\)) was found to be a promising additive to gain higher efficiencies. Beside that, binary mixtures of CO\(_2\) and alkanes have already been well investigated and thus a broad basis of experimental values including adjusted parameters for the fluid models are available. Additionally, sulfur hexafluoride (SF\(_6\)) and xenon (Xe) were considered because positive effects have already been observed several times for these components for different configurations of sCO\(_2\) power cycles [3, 11]. However, for both components no adjusted parameters for the multi-fluid mixture model are available, which makes it reasonable to consider predictive approaches in particular for these combinations. Moreover, as past work has shown that critical lines calculated with simpler mixture models may show significant deviations from experimental values (cf. e.g., [3]), this additionally encourages to extend the scope of available models also to higher order predictive methods.
With reference to the equations of state and the mixture models described in Sect. 1.1, the algorithm, implemented within the critical line routine of TREND (CRIT_LINE), was used to compare several combinations of equations of state and mixture models. In this way, the multiparameter EoS were combined with the multi-fluid mixture model, as well as with both COSMO-SAC versions mentioned previously, with [41] and without [40] its dsp extension. Furthermore, the SRK EoS was used in combination with SRK mixing rules on the one hand using adjusted values for \(k_{ij}\) and on the other hand with \(k_{ij}\) predictively set to 0 as well as with both versions of COSMO-SAC. Table 1 shows an overview of the individual model combinations as well as their abbreviated designation used for referencing within the text and the plots.
Regarding the parameters for the models, the COSMO-files, except for SF\(_6\) and xenon, have been taken from the database provided by Bell et al. [46], which is an extension of the database provided by Mullins et al. [49]. The sigma profiles for SF\(_6\) and xenon were calculated in-house based on the parameter description by Mullins et al. [49] using the Dmol\(^3\) software package. An overview of the parameters of all other models used within this work, if not named directly within the text, can be found in the appendices A to C.
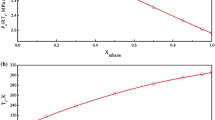
Calculated critical lines and experimental values for binary mixtures of CO\(_2\) and alkanes up to hexane. Model designation in the legend according to Table 1
Starting with the alkanes, Fig. 2 shows a comparison of the critical lines calculated with the different model combinations from methane to hexane. Herein, for model combinations not listed in the legend of the respective mixture, no valid or no converged solution could be found. Gaps and missing sections in the graphs indicate locally non-converged solutions which were discarded and therefore not show up in the plots. In addition, citations for the experimental data shown in the plots (abbreviated in the legends) can be found in tabular form in Appendix D, and, in most cases, as direct references in the text.
For CO\(_2\) + methane (CH\(_4\)), both, the multiparameter EoS with the multi-fluid mixture model (MpEoS+MFmix), as well as the SRK with the SRK mixture model and an adjusted value for \(k_{ij}\) (SRK+adjk) show good accordance with the experimental values. Whereas, starting from the critical point of pure methane, in the first third of the line there is hardly any difference between the models, clear differences can be seen from approx. 230 K onwards. Referring to the data provided by Donelli et al. [50] and Kaminishi et al. [51], within this area, the SRK even shows a slightly better agreement with the data than the combination of the multiparameter EoS and the multi-fluid mixture model, which slightly overpredicts the resulting pressure peak in the critical line. On the other hand, a larger deviation can be seen when the SRK is used predictively, without an adjusted parameter for the mixture (SRK+predk). However, none of the combinations with one of the two COSMO-SAC models provided a feasible solution.
Applied to CO\(_2\) + ethane (C\(_2\)H\(_6\)), and as shown in the upper right subplot of Fig. 2, the model combinations MpEoS+MFmix and SRK+adjk show a generally good accordance with the experimental values provided by Khazanova et al. [52], Ohgaki et al. [53], Morrison et al. [54], and Horstmann et al. [55]. Both model combinations are capable to represent the pressure-maximum azeotropic behavior of the mixture. This results in a characteristic dip of the curve towards lower temperatures while the critical temperatures of the pure substances are relatively close to each other (\(T_{\text {c,CO}_2}\approx\) 304.13 K, \(T_{\text {c,C}_2\text {H}_6}\approx\) 305.32 K). When taking a closer look at the curves which were calculated predictively, it is noticeable that the SRK without an adjusted value for \(k_{ij}\) (SRK+predk) is hardly able to represent the experimental data. As can be seen in the plot in Fig. 2, the resulting line creates almost a direct connection of the critical points of both pure fluids only showing a slight bend to lower temperatures. If, on the other hand, the SRK EoS is used with COSMO-SAC in the theoretically-based departure function, both versions of the mixture model show a significantly too strong expression of the azeotropic behavior, which is even more pronounced for COSMO-SAC-dsp (SRK+COSMOdsp) [41] compared to COSMO-SAC (SRK+COSMO) [40]. Deviations are sometimes more than 10 K compared to the experimental data. Regarding the multiparameter EoS, and similar to methane, its combination with both COSMO-SAC models does not result in a feasible solution. This was observed also for the mixtures of CO\(_2\)+propane (C\(_3\)H\(_8\)) and partially for CO\(_2\)+n-butane (C\(_4\)H\(_{10}\)) as well as CO\(_2\)+n-pentane (C\(_5\)H\(_{12}\)). A reason for the problems calculating the critical lines for these mixtures with multiparameter equations of state in combination with COSMO-SAC could be the known fact [39] that the reference equation for CO\(_2\) exhibits relatively small first maxima on subcritical isotherms in a p-\(\rho\) diagram. When the vapor-liquid equilibrium (VLE) pressure of the mixture is increasing, this can lead to problems finding a valid solution for the vapor density, as was already discussed by Jäger et al. [39]. The SRK equation of state typically exhibits higher maxima than the reference equation of state and therefore when using the SRK for the pure components in the mixture model instead of the reference equations of state, these problems finding a valid vapor density solution can be avoided.
For CO\(_2\) + propane (C\(_3\)H\(_8\)), in the middle left of Fig. 2, certain disagreements between the experimental data from the individual sources can be noted. Starting from a temperature of approx. 312 K onwards to the critical point of pure propane, the measured values by Poettmann et al. [56] and Reamer et al. [57] describe a curve at significantly higher pressures than the data from more recent sources (cf. [54, 58,59,60]). With respect to the lines calculated within this work, the multiparameter EoS combined with the multi-fluid mixture model (MpEoS+MFmix) are in good agreement with the data measured by Poettman et al. [56] and Reamer et al. [57], while the SRK EoS with an adjusted \(k_{ij}\) parameter (SRK+adjk) fits better to the measured data from the more recent sources. This in turn leads to the assumption that the models were fitted to different experimental data. Regarding the predictive calculations, as for the mixtures CO\(_2\)+methane and CO\(_2\)+ethane, the combination of multiparameter EoS and both COSMO-SAC models does not result in any feasible solution. Nevertheless, combined with the SRK EoS, both formulations can reproduce the qualitative profile of the curve. Here, however, it appears that the initial bump in the curve near the critical point of pure CO\(_2\) is more pronounced than the experimental values and the other models indicate. As before in the case of the exaggerated azeotropic behavior of CO\(_2\) and ethane, this effect is also more pronounced in COSMO-SAC-dsp than for the model which does not consider the dispersive influence. If, on the other hand, the SRK EoS is used with standard SRK mixing rules and without a fitted parameter (SRK+predk), the critical points of the pure substances are merely connected by a simple arc, without reproducing the characteristic shape of the curve. Nevertheless, at increasing propane content all curves calculated with the SRK EoS are in a relatively good agreement to each other.
For CO\(_2\) + n-butane (C\(_4\)H\(_{10}\)), both model combinations with fitted mixture parameters (MpEoS+MFmix and SRK+adjk) show good agreement with the experimental values, although the multi-fluid mixture model tends to slightly higher pressures in the curve than the SRK with an adjusted value for \(k_{ij}\). Looking at the predictive calculations using COSMO-SAC and starting from the critical point of pure n-butane, in the first half of the plot both model formulations in combination with the multiparameter EoS show a quite similar curve tending to slightly higher pressures/temperatures than the resulting lines of the adjusted models and the experimental data. From a temperature of about 370 K onwards, however, both curves bend prematurely towards the critical point of pure CO\(_2\). This is well ahead of the course indicated by the fitted models and the experimental data, leading to a trend at significantly lower pressures for the last third of the curve, including a slight dip near pure CO\(_2\), similar but not as pronounced as for CO\(_2\) + propane. More obvious, however, indicated by the gap in both plots, is that neither of the two models could close the critical lines. Thus, with the multiparameter EoS + COSMO-SAC (MpEoS+COSMO) for temperatures between approx. 330 K and 340 K and for the combination of EoS with COSMO-SAC-dsp (MpEoS+COSMOdsp) even for the temperature range between approx. 310 K and 360 K no converged solution could be achieved. If the COSMO-SAC formulations are used with the SRK EoS instead, both models are able to close the critical lines. Starting from the critical point of pure n-butane, the agreement with the experimental data is even better in the first part of the curve than the combination with the multiparameter EoS. The slight trend towards higher pressures mentioned above is only present for the non-dsp formulation [40] and not to the same extent as before. Nevertheless, the aforementioned bend towards the critical point of pure CO\(_2\) occurs even earlier and also results in a dip, which is more developed with the dsp formulation than without. It is also remarkable that the SRK combination without an adjusted parameter for \(k_{ij}\) (SRK+predk) is mostly in good agreement with the experimental data. Only in the last third of the curve, towards pure CO\(_2\), there is a clear deviation towards higher pressures.
Looking at CO\(_2\) + n-pentane (C\(_5\)H\(_{12}\)) in the lower left subplot of Fig. 2, compared to the experimental data (cf. [56, 60,61,62,63]) the combination of the highly accurate equations of state (MpEoS+MFmix) shows a notable offset to higher pressures over almost the entire range. On the other hand, when COSMO-SAC is used as a mixture model, both formulations show a significantly better reproduction of the experimental data, although still with a shift towards higher pressures in the right half of the curve. However, the line calculated with the COSMO-SAC-dsp model shows a small gap near the critical point of pure CO\(_2\), for which no converged solution could be achieved. On the other hand, the SRK with adjusted mixing parameters shows good agreement with the experimental values of Poettmann et al. [56] and Cheng et al. [61] over the whole range of the curve. Experimental data from more recent sources (cf. [60, 62, 63]) describe a somewhat flatter curve in the middle of the plot, which again leads to the assumption that the parameter \(k_{ij}\) was fitted to a different selection or only to a part of the experimental data considered here, as in the case of the multi-fluid mixture model for CO\(_2\) + propane. Even without an adjusted value for \(k_{ij}\), the SRK is in fairly good agreement with the experimental data. There is a slight overshoot to higher pressures at higher concentrations of CO\(_2\). It is also worth noting that the agreement with the experimental data is even better than when using the multiparameter EoS combined with the multi-fluid mixture model (MpEoS+MFmix). Also in conjunction with the two COSMO-SAC models, the SRK EoS is able to reproduce the qualitative shape of the critical line given by the experimental data, but with slight deviations towards lower pressures. Between the two COSMO-SAC formulations there are only small differences.
A somewhat similar result can be seen for CO\(_2\) + n-hexane (C\(_6\)H\(_{14}\)) compared to the experimental data by Gurdial et al. [62], Choi et al. [64], Liu et al. [65], and Sun et al. [66]. The combination of the adapted fluid models (MpEoS+MFmix) leads to a clear overestimation of the critical pressures, which is even more pronounced than with CO\(_2\) + n-pentane. Compared to experimental data, for example from Liu et al. [65], deviations greater than 5 MPa (at approx. 480 K) can be found. Nevertheless, also here, better results can be achieved by combining the multiparameter EoS with COSMO-SAC resulting in still notable higher pressures for larger amounts of n-hexane, changing to slightly underestimated values with decreasing intensity towards pure CO\(_2\). Herein, the dsp extension of COSMO-SAC performs slightly better than the basic formulation. Despite that, the SRK EoS with adjusted mixing rule shows a good agreement with the experimental data although, compared with the results by Liu et al. [65], locally with a slight shift to higher pressures. However, it is noteworthy that for larger amounts of n-hexane, i.e., in the temperature range of about 425 K to 480 K, the predictive models are significantly closer to the experimental values than the models with the adjusted parameters. This also applies to the use of the SRK mixing rules without adjusted value for \(k_{ij}\) (SRK+predk).
Calculated critical lines and experimental values for binary mixtures of CO\(_2\) + SF\(_6\) and CO\(_2\) + Xe. Model designation in the legend according to Table 1
Finally, Fig. 3 shows the critical lines for CO\(_2\) + SF\(_6\) and for CO\(_2\) + Xe. As noted above, there are no fitted parameters for the multi-fluid mixture model for either mixture, so predictive methods are of particular interest here. Starting with CO\(_2\) + SF\(_6\), critical lines were calculated with both COSMO-SAC formulations and SRK mixing rules with and without adjusted values for \(k_{ij}\). As in the work of Hsieh et al. [41] no group contribution for SF\(_6\) is available to calculate the dispersive coefficient for COSMO-SAC-dsp, its value was adjusted manually in this work (to \(\varepsilon /k_\text {B}=164.0\)) to achieve a good agreement with the critical property data provided by Krichevskii et al. [67] and Diefenbacher et al. [68] in combination with the SRK EoS. Thus, as can be seen in the left plot in Fig. 3, the SRK EoS combined with COSMO-SAC-dsp (SRK+COSMOdsp) can reproduce the qualitative shape of the critical line including its pressure maximum azeotropic behavior albeit with some deviations from the experimental values in the lower half of the curve. In combination with the multiparameter EoS, however, the agreement with the available data is worse. While mixtures with a high CO\(_2\) fraction are still well represented, the critical curve bends from about 303 K towards an almost direct connection to the critical point for pure SF\(_6\), ignoring the more pronounced dip to lower temperatures. Hereby, it should be noted that even a further adjustment of the dispersion parameter for the combination (MpEoS+COSMOdsp) did not lead to an accurate representation of the experimental data. If, on the other hand, the dispersive part is not taken into account, i.e., the model COSMO-SAC [40] is used, neither applying the SRK nor multiparameter EoS for the pure fluids leads to a good agreement with the critical line. As can be seen in Fig. 3, the curvature of the critical line in both cases results in a sweeping arc towards higher, rather than lower, temperatures. Instead of a pressure minimum azeotrope, the critical line thus incorrectly represents a pressure maximum azeotrope. In addition, this effect is more pronounced for the multiparameter EoS than for the SRK. Again, there are convergence problems when using the model combination (MpEoS+COSMO) leading to gaps within the result plots. Returning to the significantly better solution of the SRK EoS using COSMO-SAC-dsp with the dispersion coefficient adjusted in this work, it is noticeable that the use of the SRK mixing rules with an adjusted \(k_{ij}\) does not give a better result in this case. Both with and without the adjusted value for \(k_{ij}\), the direction of the curve is correctly reproduced, but only with a clear deviation from the experimental data.
For CO\(_2\) + xenon (Xe), only 2 model combinations were able to provide a viable solution for the critical line. As shown in Fig. 3, the predictive application of the SRK almost directly connects the critical points of both pure substances. The azeotropic behavior of the mixture is not predicted by the SRK. Since no adjusted parameter for \(k_{ij}\) was available, it was adjusted manually, analogously to the dispersion parameter of the COSMO-SAC-dsp model for CO\(_2\) + SF\(_6\), targeting a good agreement of the calculated critical line with the available experimental data. As can be seen in Fig. 3, the SRK with an adjusted value of \(k_{\text {CO}_2,\text {Xe}}=0.1410\) results in a very good representation of the experimental data by Martynets et al. [69] Ribeiro et al. [70]. The characteristic curve of the pressure maximum azeotrope is correctly reproduced in both position and direction. As there were no parameters available for CO\(_2\) and Xenon for the multi-fluid mixture model, this model could not be used for the calculation of the critical line. Moreover, all combinations with COSMO-SAC, even with manual adjustment of the dispersion coefficient as for CO\(_2\) + SF\(_6\), did not lead to any converged solution.
For further comparison, additional plots (x-\(T_\text {c}\), x-\(p_\text {c}\)) of the critical lines shown in this paragraph, can be found in the supplementary material.
4 Conclusion
Within this work, a method for the calculation of critical points of fluid mixtures is presented. Based on the calculation procedure by Heidemann and Khalil [19] and subsequent works i.e., by Michelsen et al. [30] as well as Stockfleth and Dohrn [24], a fully numerical algorithm was created with an emphasis on numerical robustness promoting a fast and reliable generation of results. This includes, for example, the use of more complex differentiation schemes to predict the next iteration steps, the use of dynamic relaxation factors to limit overshooting in early iteration phases as well as estimation methods to guess starting values. Implemented into the thermodynamic property software TREND, with its fluid modeling capacities, the algorithm allows the calculation of critical points and lines for a flexible combination of different equations of state and mixture models also including predictive approaches. Hereby, the consistent use of standard TREND routines enables the use of all fluid models implemented in TREND including a direct compatibility with possible future extensions. Against the background of an increasing relevance of supercritical CO\(_2\) mixtures in energy applications, the routine was applied to a selection of binary CO\(_2\)-mixtures recently considered for sCO\(_2\) power cycles. Herein, the results of the routine for different combinations of EoS and mixture models were compared against experimental values with a special emphasis on predictive mixture models, such as the combination of the multi-fluid mixture model with the excess Gibbs energy (\(g^\text {E}\)) model COSMO-SAC which is presented for the first time in this article.
By using the algorithm, critical lines could be calculated for all selected mixtures. However, with reference to the different model configurations, remarkable variations could be observed within the results. Generally, combinations using parameters which are readily adapted to the mixture, such as the multiparameter EoS with the multi-fluid mixture model, or the SRK with an adapted value for \(k_{ij}\), in most of the cases were in relatively good agreement with the experimental data. Thereby, it was apparent that the SRK often fits even better to the experimental values than the multiparameter EoS combined with the multi-fluid mixture model. However, note that the GERG-2008 model by Kunz et al. [27, 33] was developed for typical natural gas mixtures with CO\(_2\) being a minor component and therefore, the CO\(_2\) mixtures considered in this work are not the main focus of the GERG-2008 model. Additionally, results such as those for CO\(_2\)+propane indicate that a significant influence may be expected from different datasets originally used to adjust the model parameters. Connected to this, the influence of adjusted parameters could be demonstrated for the mixture CO\(_2\)+xenon. By manually adjusting the \(k_{ij}\) parameter, a good agreement with the experimental data could be achieved, whereas the predictive use of the SRK did not yield satisfactory results. In general, the predictive applicability of SRK mixing rules was found to be quite limited, as more complex effects such as an azeotropic behavior are not or only partially captured.
In contrast, better predictive results could be achieved by combining the multi-fluid mixture model with the excess Gibbs energy model COSMO-SAC and its extension COSMO-SAC-dsp. Resulting predictions of critical lines were partially even more accurate than those of the GERG-2008 model. Especially for mixtures containing alkanes with increasing chain length, such as CO\(_2\)+pentane and CO\(_2\)+hexane, a good agreement with the experimental values could be found. For mixtures containing only small molecules, such as CO\(_2\)+ethane or CO\(_2\)+xenon, both COSMO-SAC formulations in conjunction with the multi-fluid mixture model show only limited applicability. However, this was expected, as COSMO-SAC was originally developed for long-chain complex molecules and its parameters have rather not been fitted to small molecules. Apart from that, regarding its dsp extension, a significant influence of the dispersive effects could be noted for some cases. For mixtures containing shorter alkanes, the dsp extension partially leads to an overprediction of single effects, like e.g., the azeotropic behavior of CO\(_2\)+ethane, while it tends to an improved representation of the experimental values for mixtures containing longer-chain alkanes. Moreover, a huge influence was apparent in particular for CO\(_2\)+SF\(_6\), where a good agreement could be achieved by manually adjusting the dispersion parameter, while the basic model, without consideration of dispersive effects, did not provide a good solution.
Overall, it can be stated that the quality and reliability of results for calculated critical lines depends significantly on the combination of EoS and mixture models. While, as expected, models adapted to the experimental data usually lead to better results than predictive approaches it could be shown that for some cases predictive approaches could provide a powerful alternative, especially if no adapted values are available. Specifically with regard to the results using COSMO-SAC, a certain potential for CO\(_2\) mixtures containing higher order alkanes could be identified, which, referring to CO\(_2\) applications in energy technology, has a quite high practical relevance e.g., due to impurification of the CO\(_2\) by lubricants or other residual substances within single components.
Abbreviations
- a :
-
Parameter in the Soave-Redlich-Kwong EoS
- A (J):
-
Total Helmholtz free energy
- a (J mol-1):
-
Molar Helmholtz free energy
- A (Å2):
-
Molecular surface area
- A :
-
Parameter in the COSMO-SAC-dsp model
- b :
-
Parameter in the Soave-Redlich-Kwong EoS
- b :
-
Co-volume within COSMO-SAC
- f :
-
Symbolic function name
- \(f', f'', f'''\) :
-
First, second and third derivative of f
- \(f_i\) (Pa):
-
Fugacity of component i
- \(f_T\) :
-
Factor to alter the guess for \(T_\text {c}\) within the calculation algorithm
- \(f_v\) :
-
Factor to alter the guess for \(v_\text {c}\) within the calculation algorithm
- F :
-
Parameter of the departure function
- G (J):
-
Total Gibbs free energy
- g (J·mol−1):
-
Molar Gibbs energy
- h :
-
Finite differentiation step size / finite increment
- k :
-
Differentiation step multiple
- \(k_{ij}\) :
-
Binary interaction parameter in the SRK mixing rules for the components i and j
- \(k_\text {B}\) (J·K−1):
-
Boltzmann constant, \(k_\text {B}=1.380649\cdot 10^{-23}\;\text{J}\cdot\text{K}^{-1}\)
- \({\varvec{n}}\) (mol):
-
Vector of mole numbers
- \(\varvec{\tilde{n}}\) (mol):
-
Normalized mole number vector n with its component j varied by kh
- \(\varvec{\tilde{\tilde{n}}}\) (mol):
-
Normalized vector of mole numbers with a simultaneous variation of all components
- \(\Delta {\varvec{n}}\) (mol):
-
Vector with mole number differences for each component
- N :
-
Total number of components (in a mixture)
- p (Pa):
-
Pressure
- \(p(\sigma )\) :
-
Sigma profile
- \(q_{ij}\) :
-
Partial composition derivatives of the Helmholtz free energy
- \(q^*_{ij}\) :
-
Partial composition derivatives of Q
- \({\varvec{Q}}\) :
-
Matrix with partial composition derivatives \(q_{ij}\) of the Helmholtz free energy
- \(\varvec{Q^*}\) :
-
Matrix with partial composition derivatives \(q^*_{ij}\) of \({\varvec{Q}}\)
- R (J·mol−1·K−1):
-
Universal gas constant, \(R\approx 8.31446\;\text{J}\cdot\text{mol}^{-1}\cdot\text{K}^{-1}\)
- s :
-
Change in the mixture’s composition
- T (K):
-
Temperature
- v (m\(^3\)·mol−1):
-
Molar volume
- V (m\(^3\)·mol−1):
-
Total volume
- \(\tilde{v}\) (m\(^3\)·mol−1):
-
Corrected molar volume v for \(\varvec{\tilde{n}}\) at a constant total volume V
- \(\tilde{\tilde{v}}\) (m3·mol−1):
-
Corrected molar volume v for a simultaneous variation of all mole numbers at a constant total volume V
- x (mol·mol−1):
-
Molar fraction
- \(\tilde{x}\) (mol·mol−1):
-
Corrected molar fraction for \(\varvec{\tilde{n}}\) at a constant total volume V
- \(\tilde{\tilde{x}}\) (mol·mol−1):
-
Corrected molar fraction x for a simultaneous variation of all mole numbers at a constant total volume V
- \(\varvec{\textbf{x}}\) (mol·mol−1):
-
Composition vector with its sum=1, containing molar fractions \(x_i\) for each component i
- y (kg·kg−1):
-
Mass fraction
- \(\alpha\) :
-
Dimensionless Helmholtz energy
- \(\beta\) :
-
Adjustable parameter of the multi-fluid mixture model
- \(\gamma\) :
-
Adjustable parameter of the multi-fluid mixture model
- \(\Gamma\) :
-
Segment activity coefficient
- \(\delta\) :
-
Reduced density, \(\delta =\rho/\rho_\text {c}\)
- \(\varepsilon\) :
-
Dispersion parameter in COSMO-SAC-dsp
- \(\vartheta\) (\(^{\circ }\)C):
-
Temperature
- \(\phi _i\) :
-
Fugacity coefficient
- \(\phi _{\text {c},i}\) :
-
Critical volume fraction of component i
- \(\psi\) :
-
Relaxation factor
- \(\rho\) (mol · m- 3):
-
Molar density
- \(\sigma\) (e Å\(^{-2}\)):
-
Screening charge density
- \(\tau\) :
-
Reciprocal reduced temperature, \(\tau =T_\text{c}/T\)
- \(\omega\) :
-
Acentric factor
- 0:
-
Initial state
- c:
-
Critical property
- i, j, k:
-
Component indices
- m:
-
Total number of components / Total amount of surface elements (COSMO-SAC)
- mix:
-
Referring to the mixture
- n:
-
Iteration counter
- o:
-
Referring to the pure fluid property
- r:
-
Reducing property
- ref:
-
Reference state
- 0:
-
Ideal gas property
- nhb:
-
Non-hydrogen bondings
- r:
-
Residual property
- E:
-
Excess property
- OH:
-
Hydroxyl groups
- OT:
-
Other atoms
- COSMO:
-
Conductor-like screening model
- COSMO-RS:
-
COSMO for real solvents
- COSMO-SAC:
-
COSMO segment activity coefficient
- COSMO-SAC-dsp:
-
COSMO-SAC including an additional dispersive influence
- CSP:
-
Concentrated solar power
- EoS:
-
Equation(s) of State
- PR:
-
Peng-Robinson (Equation of State)
- PSRK:
-
Predictive Soave-Redlich-Kwong (Equation of State)
- SAFT:
-
Statistical associating fluid theory
- SRK:
-
Soave-Redlich-Kwong (Equation of State)
- TREND:
-
Thermodynamic Reference and Engineering Data (Software)
References
G. Brunner, Applications of supercritical fluids. Annu. Rev. Chem. Biomol. Eng. 1, 321–342 (2010). https://doi.org/10.1146/annurev-chembioeng-073009-101311
F. Crespi, P. Rodríguez de Arriba, D. Sánchez, A. Ayub, G. Di Marcoberardino, C.M. Invernizzi et al., Thermal efficiency gains enabled by using CO2 mixtures in supercritical power cycles. Energy 238, 121899 (2022). https://doi.org/10.1016/j.energy.2021.121899
S. Rath, E. Mickoleit, U. Gampe, C. Breitkopf, A. Jäger, Systematic analysis of additives on the performance parameters of sCO2 cycles and their individual effects on the cycle characteristics. Energy 252, 123957 (2022). https://doi.org/10.1016/j.energy.2022.123957
S. Rath, C. Breitkopf, U. Gampe, A. Jäger, Impact of Selective Admixture of Additives to Carbon Dioxide on the Size of sCO2 Power Cycle Key Components. In: Proceedings of the 7th International Supercritical CO2 Power Cycles Symposium. San Antonio, Texas, USA;( 2022)
R. Valencia-Chapi, L. Coco-Enríquez, J. Muñoz-Antón, Supercritical CO2 mixtures for advanced brayton power cycles in line-focusing solar power plants. Appl. Sci. 10, 55 (2019). https://doi.org/10.3390/app10010055
Q. Zhao, M. Mecheri, T. Neveux, R. Privat, J.N. Jaubert, Selection of a proper equation of state for the modeling of a supercritical CO\({_{2}}\) Brayton cycle: consequences on the process design. Ind. Eng. Chem. Res. 56, 6841–6853 (2017). https://doi.org/10.1021/acs.iecr.7b00917
E. Mickoleit, C. Breitkopf, A. Jäger, Influence of equations of state and mixture models on the design of a refrigeration process. Int. J. Refrig. 121, 193–205 (2021). https://doi.org/10.1016/j.ijrefrig.2020.10.017
S. Rath, U. Gampe, A. Jäger, Influence of the Equation of State on the Design of sCO2-power Cycles. Conference Proceedings of the 5th European sCO2 Conference for Energy Systems: March 14-16. Apr;2023:p. 66. (2023) https://doi.org/10.17185/DUEPUBLICO/77268
P. Arce, M. Aznar, Modeling of critical lines and regions for binary and ternary mixtures using non-cubic and cubic equations of state. J. Supercrit. Fluids 24, 1–26 (2007). https://doi.org/10.1016/j.supflu.2007.02.001
G. Manzolini, M. Binotti, D. Bonalumi, C. Invernizzi, P. Iora, CO2 mixtures as innovative working fluid in power cycles applied to solar plants. Techno-econ. Assess. Sol. Energy 181, 530–544 (2019). https://doi.org/10.1016/j.solener.2019.01.015
W.S. Jeong, Y.H. Jeong, Performance of supercritical Brayton cycle using CO2-based binary mixture at varying critical points for SFR applications. Nucl. Eng. Des. 262, 12–20 (2013). https://doi.org/10.1016/j.nucengdes.2013.04.006
TM. Conboy, Wright SA, Lewis TG. CO2-based Mixtures as Working Fluids for Geothermal Turbines.; SAND2012-4905, 1049477 (2012)
J.W. Gibbs, ART. LII.—on the equilibrium of heterogeneous substances. Am. J. Sci. Arts. 16, 441–459 (1878)
O. Redlich, A.T. Kister, On the thermodynamics of solutions. VII. Critical properties of mixtures. J. Chem. Phys. 36, 2002–2009 (1962). https://doi.org/10.1063/1.1732819
R.R. Spear, R.L. Robinson, K.C. Chao, Critical states of mixtures and equations of state. Ind. Eng. Chem. Fundam. 8 2–8 (1969). https://doi.org/10.1021/i160029a002
R.R. Spear, R.L. Robinson, K.C. Chao, Critical states of ternary mixtures and equations of state. Ind. Eng. Chem. Fundam. 10, 588–592 (1971). https://doi.org/10.1021/i160040a008
P.H. van Konynenburg, R.L. Scott, Critical lines and phase equilibria in binary van Der Waals mixtures. Philos. Trans. R. Soc. Lond. Ser. A 298, 495–540 (1980). https://doi.org/10.1098/rsta.1980.0266
L.E. Baker, K.D. Luks, Critical point and saturation pressure calculations for multipoint systems. Soc. Pet. Eng. J. 20, 15–24 (1980). https://doi.org/10.2118/7478-PA
R.A. Heidemann, A.M. Khalil, The calculation of critical points. AIChE J. 26, 769–779 (1980). https://doi.org/10.1002/aic.690260510
M.L. Michelsen, R.A. Heidemann, Calculation of critical points from cubic two-constant equations of state. AIChE J. 27, 521–523 (1981). https://doi.org/10.1002/aic.690270326
P. Kolář, K. Kojima, Prediction of critical points in multicomponent systems using the PSRK group contribution equation of state. Fluid Phase Equilib. 118, 175–200 (1996). https://doi.org/10.1016/0378-3812(95)02850-1
T. Holderbaum, J. Gmehling, PSRK: a group contribution equation of state based on UNIFAC. Fluid Phase Equilib. 70, 251–265 (1991)
S. Horstmann, A. Jabloniec, J. Krafczyk, K. Fischer, J. Gmehling, PSRK group contribution equation of state: comprehensive revision and extension IV, including critical constants and \(\alpha\)-function parameters for 1000 components. Fluid Phase Equilib. 227, 157–164 (2005). https://doi.org/10.1016/j.fluid.2004.11.002
R. Stockfleth, R. Dohrn, An algorithm for calculating critical points in multicomponent mixtures which can easily be implemented in existing programs to calculate phase equilibria. Fluid Phase Equilib. 145, 43–52 (1998). https://doi.org/10.1016/S0378-3812(97)00225-2
I.H. Bell, A. Jäger, Calculation of critical points from Helmholtz-energy-explicit mixture models. Fluid Phase Equilib. 433, 159–173 (2017). https://doi.org/10.1016/j.fluid.2016.10.030
U.K. Deiters, I.H. Bell, Calculation of critical curves of fluid mixtures through solution of differential equations. Ind. Eng. Chem. Res. 59, 19062–19076 (2020). https://doi.org/10.1021/acs.iecr.0c03667
O. Kunz, R. Klimeck, W. Wagner, M. Jaeschke, The Gerg-2004 Wide Range Equation of State for Natural Gases and Other Mixtures GERG TM15 2007 (VDI-Verl, Düsseldorf, 2007)
U.K. Deiters, I.H. Bell, Unphysical critical curves of binary mixtures predicted with GERG models. Int. J. Thermophys. 41, 169 (2020). https://doi.org/10.1007/s10765-020-02743-3
R. Span, R. Beckmüller, S. Hielscher, A. Jäger, E. Mickoleit, T. Neumann, et al. TREND. Thermodynamic Reference and Engineering Data 5.0. Lehrstuhl für Thermodynamik, Ruhr-Universität Bochum
M.L. Michelsen, Calculation of phase envelopes and critical points for multicomponent mixtures. Fluid Phase Equilib. 4, 1–10 (1980). https://doi.org/10.1016/0378-3812(80)80001-X
R. Span, Multiparameter Equations of State: An Accurate Source of Thermodynamic Property Data (Springer, Berlin, 2000)
I.H. Bell, A. Jäger, Helmholtz energy transformations of common cubic equations of state for use with pure fluids and mixtures. J. Res. Nat. Inst. Stand. Technol. 121, 238 (2016). https://doi.org/10.6028/jres.121.011
O. Kunz, W. Wagner, The GERG-2008 wide-range equation of state for natural gases and other mixtures: an expansion of GERG-2004. J. Chem. Eng. Data 57, 3032–3091 (2012). https://doi.org/10.1021/je300655b
E.W. Lemmon, R. Tillner-Roth, A Helmholtz energy equation of state for calculating the thermodynamic properties of fluid mixtures. Fluid Phase Equilib. 165, 1–21 (1999)
J. Gernert, R. Span, EOS-CG: a Helmholtz energy mixture model for humid gases and CCS mixtures. J. Chem. Thermodyn. 93, 274–293 (2016). https://doi.org/10.1016/j.jct.2015.05.015
S. Herrig, New Helmholtz-Energy Equations of State for Pure Fluids and CCS-Relevant Mixtures (Bochum, Germany, 2018)
R. Klimeck, Entwicklung Einer Fundamentalgleichung Für Erdgase Für Das Gas- Und Flüssigkeitsgebiet Sowie Das Phasengleichgewicht. Bochum, Germany
A. Jäger, E. Mickoleit, C. Breitkopf, A combination of multi-fluid mixture models with COSMO-SAC. Fluid Phase Equilib. 476, 147–156 (2018). https://doi.org/10.1016/j.fluid.2018.08.004
A. Jäger, I.H. Bell, C. Breitkopf, A theoretically based departure function for multi-fluid mixture models. Fluid Phase Equilib. 469, 56–69 (2018). https://doi.org/10.1016/j.fluid.2018.04.015
C.M. Hsieh, S.I. Sandler, S.T. Lin, Improvements of COSMO-SAC for vapor- liquid and liquid- liquid equilibrium predictions. Fluid Phase Equilib. 297, 90–97 (2010). https://doi.org/10.1016/j.fluid.2010.06.011
C.M. Hsieh, S.T. Lin, J. Vrabec, Considering the dispersive interactions in the COSMO-SAC model for more accurate predictions of fluid phase behavior. Fluid Phase Equilib. 367, 109–116 (2014). https://doi.org/10.1016/j.fluid.2014.01.032
S.T. Lin, S.I. Sandler, A priori phase equilibrium prediction from a segment contribution solvation model. Ind. Eng. Chem. Res. 41, 899–913 (2002). https://doi.org/10.1021/ie001047w
S.T. Lin, S.I. Sandler, A priori phase equilibrium prediction from a segment contribution solvation model—additions and corrections. Ind. Eng. Chem. Res. 43, 1322–1322 (2004). https://doi.org/10.1021/ie0308689
A. Klamt, G. Schüürmann, COSMO: a new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. Perkin Trans. 2, 799–805 (1993). https://doi.org/10.1039/P29930000799
A. Klamt, Conductor-like screening model for real solvents: a new approach to the quantitative calculation of solvation phenomena. J. Phys. Chem. 99, 2224–2235 (1995)
I.H. Bell, E. Mickoleit, C.M. Hsieh, S.T. Lin, J. Vrabec, C. Breitkopf et al., A benchmark open-source implementation of COSMO-SAC. J. Chem. Theory Comput. 16, 2635–2646 (2020). https://doi.org/10.1021/acs.jctc.9b01016
C.C. Li, Critical temperature estimation for simple mixtures. Can. J. Chem. Eng. 49, 709–710 (1971). https://doi.org/10.1002/cjce.5450490529
RL. Scott, PHv. Konynenburg, Static properties of solutions. Van Der Waals and related models for hydrocarbon mixtures. Discussions Faraday Soc. 49(0):87–97 (1970)
E. Mullins, R. Oldland, Y.A. Liu, S. Wang, S.I. Sandler, C.C. Chen et al., Sigma-profile database for using COSMO-based thermodynamic methods. Ind. Eng. Chem. Res. 45, 4389–4415 (2006). https://doi.org/10.1021/ie060370h
H.G. Donnelly, D.L. Katz, Phase equilibria in the carbon dioxide-methane system. Ind. Eng. Chem. 46, 511–517 (1954). https://doi.org/10.1021/ie50531a036
Gi. Kaminishi, T. Toriumi, Vapor-liquid equilibria in the systems: CO2-CO, CO2-CO-H2 and CO2-CH4. Rev. Phys. Chem. Japan 38, 79–84 (1968)
N.E. Khazanova, L.S. Lesnevskaya, A.V. Zakharova, Liquid-vapor equilibrium in the system ethane + carbon dioxide. Khim Prom. 42, 364 (1966)
K. Ohgaki, T. Katayama, Isothermal vapor-liquid equilibrium data for the ethane-carbon dioxide system at high pressures. Fluid Phase Equilib. 1, 27–32 (1977). https://doi.org/10.1016/0378-3812(77)80023-X
G. Morrison, J.M. Kincaid, Critical point measurements on nearly polydisperse fluids. AIChE J. 30, 257–262 (1984). https://doi.org/10.1002/aic.690300213
S. Horstmann, K. Fischer, J. Gmehling, P. Kolář, Experimental determination of the critical line for (carbon dioxide + ethane) and calculation of various thermodynamic properties for (carbon dioxide +n-alkane) using the PSRK model. J. Chem. Thermodyn. 32, 451–464 (2000). https://doi.org/10.1006/jcht.2000.0611
F.H. Poettmann, D.L. Katz, Phase behavior of binary carbon dioxide-paraffin systems. Ind. Eng. Chem. 37, 847–853 (1945). https://doi.org/10.1021/ie50429a017
H.H. Reamer, B.H. Sage, W.N. Lacey, Phase equilibria in hydrocarbon systems. Volumetric and phase behavior of the propane-carbon dioxide system. Ind. Eng. Chem. 43, 2515–2520 (1951). https://doi.org/10.1021/ie50503a035
J.G. Roof, J.D. Baron, Critical loci of binary mixtures of propane with methane, carbon dioxide, and nitrogen. J. Chem. Eng. Data 12, 292–293 (1967). https://doi.org/10.1021/je60034a003
V.G. Niesen, J.C. Rainwater, Critical locus, (vapor + liquid) equilibria, and coexisting densities of (carbon dioxide + propane) at temperatures from 311 K to 361 K. J. Chem. Thermodyn. 22, 777–795 (1990). https://doi.org/10.1016/0021-9614(90)90070-7
N. Juntarachat, S. Bello, R. Privat, J.N. Jaubert, Validation of a new apparatus using the dynamic method for determining the critical properties of binary gas/gas mixtures. J. Chem. Eng. Data 58, 671–676 (2013). https://doi.org/10.1021/je301209u
H. Cheng, M.E. Pozo DeFernandez, J.A. Zollweg, W.B. Streett, Vapor-liquid equilibrium in the system carbon dioxide + n-pentane from 252 to 458 K at pressures to 10 MPa. J. Chem. Eng. Data 34, 319–323 (1989). https://doi.org/10.1021/je00057a018
GS. Gurdial, NR. Foster, J. Yun, KD. Tilly, Phase Behaviour of Supercritical Fluid-Entrainer Systems. In: Kiran E, Brennecke JF, editors. Supercritical Fluid Engineering Science: Fundamentals and Applications. vol. 514 of ACS Symposium Series. Washington, DC: American Chemical Society; 1992. p. 34–45
J. Chen, W. Wu, B. Han, L. Gao, T. Mu, Z. Liu et al., Phase behavior, densities, and isothermal compressibility of CO \({_{2}}\) + pentane and CO \({_{2}}\) + acetone systems in various phase regions. J. Chem. Eng. Data 48, 1544–1548 (2003). https://doi.org/10.1021/je034087q
E.J. Choi, S.D. Yeo, Critical properties for carbon dioxide + n -alkane mixtures using a variable-volume view cell. J. Chem. Eng. Data 43, 714–716 (1998). https://doi.org/10.1021/je9800297
J. Liu, Z. Qin, G. Wang, X. Hou, J. Wang, Critical properties of binary and ternary mixtures of hexane + methanol, hexane + carbon dioxide, methanol + carbon dioxide, and hexane + carbon dioxide + methanol. J. Chem. Eng. Data 48, 1610–1613 (2003). https://doi.org/10.1021/je034127q
Y. Sun, Y. Li, J. Zhou, R. Zhu, Y. Tian, Experimental determination and calculation of the critical curves for the binary systems of CO2 containing ketone, alkane, ester and alcohol, respectively. Fluid Phase Equilib. 307, 72–77 (2011). https://doi.org/10.1016/j.fluid.2011.05.005
I.R. Krichevskii, Thermodynamics of critical phoenomena in binary infinitely dilute solutions. Zh. Fiz. Khim. 41, 2438–69 (1967)
A. Diefenbacher, M. Türk, Critical (p, \(\rho\), T) Properties of CH2F2, xCO2+(1–x) SF6, xSF6+(1–x) CH2F2, and xCHF3+(1–x) CH2F2. J. Chem. Thermodyn. 31, 905–919 (1999). https://doi.org/10.1006/jcht.1999.0500
V.G. Martynets, N.V. Kuskova, E.V. Matizen, V.F. Kukarin, Critical line of (xenon + carbon dioxide). J. Chem. Thermodyn. 31, 191–195 (1999). https://doi.org/10.1006/jcht.1998.0438
N. Ribeiro, T. Casimiro, C. Duarte, M. Nunes da Ponte, A. Aguiar-Ricardo, M. Poliakoff, Vapor-liquid equilibrium and critical line of the CO\({_{2}}\) + Xe system. Critical behavior of CO\({_{2}}\) + Xe versus CO\({_{2}}\) + n-alkanes. J. Phys. Chem. B 104, 791–795 (2000)
R. Span, W. Wagner, A new equation of state for carbon dioxide covering the fluid region from the triple-point temperature to 1100 K at pressures up to 800 MPa. J. Phys. Chem. Ref. Data 25, 1509–1596 (1996). https://doi.org/10.1063/1.555991
D. Bücker, W. Wagner, A reference equation of state for the thermodynamic properties of ethane for temperatures from the melting line to 675 K and pressures up to 900 MPa. J. Phys. Chem. Ref. Data 35(1), 205–266 (2006). https://doi.org/10.1063/1.1859286
U. Setzmann, W. Wagner, A new equation of state and tables of thermodynamic properties for methane covering the range from the melting line to 625 K at pressures up to 100 MPa. J. Phys. Chem. Ref. Data 20, 1061–1155 (1991). https://doi.org/10.1063/1.555898
D. Bücker, W. Wagner, Reference equations of state for the thermodynamic properties of fluid phase N-butane and isobutane. J. Phys. Chem. Ref. Data 35, 929–1019 (2006). https://doi.org/10.1063/1.1901687
E.W. Lemmon, I.H. Bell, M.L. Huber, M.O. McLinden, REFPROP, NIST Standard Reference Database 23 Version 10.0. Boulder, USA
E.W. Lemmon, M.O. McLinden, W. Wagner, Thermodynamic properties of propane. III. A reference equation of state for temperatures from the melting line to 650 K and pressures up to 1000 MPa. J. Chem. Eng. Data 54, 3141–3180 (2009). https://doi.org/10.1021/je900217v
C. Guder, W. Wagner, A reference equation of state for the thermodynamic properties of sulfur hexafluoride (SF\({_{6}}\)) for temperatures from the melting line to 625 K and pressures up to 150 MPa. J. Phys. Chem. Ref. Data 38, 33–94 (2009)
E.W. Lemmon, R. Span, Short fundamental equations of state for 20 industrial fluids. J. Chem. Eng. Data 51, 785–850 (2006). https://doi.org/10.1021/je050186n
G. Soave, S. Gamba, L.A. Pellegrini, SRK Equation of state: predicting binary interaction parameters of hydrocarbons and related compounds. Fluid Phase Equilib. 299, 285–293 (2010). https://doi.org/10.1016/j.fluid.2010.09.012
H. Li, J. Yan, Evaluating cubic equations of state for calculation of vapor- liquid equilibrium of CO2 and CO2-mixtures for CO2 capture and storage processes. Appl. Energy 86, 826–836 (2009). https://doi.org/10.1016/j.apenergy.2008.05.018
A.S. Vasilyev, T.N. Gartman, Calculating the critical properties of multicomponent mixtures using the Soave-Redlich-Kwong (SRK) and Peng-Robinson (PR) equations of state, based on the binary interaction parameters. Russ. J. Phys. Chem. A 93, 620–624 (2019). https://doi.org/10.1134/S0036024419040290
I.R. Krichevskii, S.R. Khusainov, L.A. Makarevich, Osmotic pressure of dilute critical phase. Dokl. Akad. Nauk SSSR 206, 1388–12390 (1972)
R.H. Olds, H.H. Reamer, B.H. Sage, W.N. Lacey, The N-butane-carbon dioxide system. Ind. Eng. Chem. 41, 475–482 (1949). https://doi.org/10.1021/ie50471a011
J.J.C. Hsu, N. Nagarajan, R.L. Robinson, Equilibrium phase compositions, phase densities, and interfacial tensions for carbon dioxide + hydrocarbon systems. 1. Carbon dioxide + n-butane. J. Chem. Eng. Data 30, 485–491 (1985). https://doi.org/10.1021/je00042a036
S.C. Hwang, H.M. Lin, P.S. Chappelear, R. Kobayashi, Dew point study in the vapor-liquid region of the methane-carbon dioxide system. J. Chem. Eng. Data 21, 493–497 (1976). https://doi.org/10.1021/je60071a019
M.E. Pozo DeFernandez, J.A. Zollweg, W.B. Streett, Vapor-liquid equilibrium in the binary system carbon dioxide + n-butane. J. Chem. Eng. Data 34, 324–328 (1989). https://doi.org/10.1021/je00057a019
Acknowledgements
Special thanks to E. Mickoleit for creating the sigma profiles for sulfur hexafluoride (SF\(_6\)) and xenon (Xe).
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors have received no funding for the research, authorship, or publication of this work.
Author information
Authors and Affiliations
Contributions
S.R.: Conceptualization, Writing the original draft, Methodology, Software, Visualization; U.G.: Writing, review & editing, Supervision; A.J.: Conceptualization, Writing the original draft, Methodology, Software, Supervision.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Special Issue in Honor of Professor Roland Span’s 60th Birthday.
Supplementary Information
Below is the link to the electronic supplementary material.
Appendices
A Multiparameter equations of state used for calculating critical lines
Substance (IUPAC name) | Chemical formula | References | Substance name used in this work |
|---|---|---|---|
carbon dioxide | CO\(_2\) | [71] | co2 |
ethane | C\(_2\)H\(_6\) | [72] | ethane |
methane | CH\(_4\) | [73] | methane |
n-butane | C\(_4\)H\(_{10}\) | [74] | butane |
n-hexane | C\(_6\)H\(_{14}\) | [75] | hexane |
n-pentane | C\(_5\)H\(_{12}\) | [75] | pentane |
propane | C\(_3\)H\(_8\) | [76] | propane |
sulfur hexafluoride | SF\(_6\) | [77] | sf6 |
xenon | Xe | [78] | xenon |
B Parameters for the multi-fluid mixture model used for calculating critical lines
C Parameters for the SRK used for calculating critical lines
Substance | \(T_\text {c}\) in K | \(p_\text {c}\) in MPa | \(\omega\) | \(k_{ij}\) for mixture with CO\(_{2}\). |
|---|---|---|---|---|
(\(n{-}\))butane | 425.1250 | 3.7960 | 0.201 | 0.13220 [79] |
CO\(_2\) | 304.1282 | 7.3773 | 0.224 | – |
ethane | 305.3220 | 4.8722 | 0.100 | 0.13360 [79] |
(\(n{-}\))hexane | 507.8200 | 3.0429 | 0.299 | 0.13150 [79] |
methane | 190.5640 | 4.5992 | 0.011 | 0.10500 [80] |
(\(n{-}\))pentane | 469.7000 | 3.3710 | 0.251 | 0.13160 [79] |
propane | 369.8900 | 4.2512 | 0.152 | 0.13290 [79] |
SF\(_6\) | 318.7232 | 3.7550 | 0.210 | 0.02567 [81] |
xenon | 289.7330 | 5.8420 | 0.004 | 0.14100\(^{*}\) |
D References to the experimental data used to compare the results
Abbrev. in the plots | References | Abbrev. in the plots | References |
|---|---|---|---|
chen2003 | [63] | krichevskii1972 | [82] |
cheng1989 | [61] | liu2003 | [65] |
choi1998 | [64] | martynets1999 | [69] |
diefenbacher1999 | [68] | morrison1984 | [54] |
donnelly1954 | [50] | niesen1990 | [59] |
gurdial1992 | [62] | ohgaki1977 | [53] |
horstmann2000 | [55] | olds1949 | [83] |
hsu1985 | [84] | poettmann1945 | [56] |
hwang1976 | [85] | pozodefernandez1989 | [86] |
juntarachat2013 | [60] | reamer1951 | [57] |
kaminishi1968 | [51] | ribeiro2000 | [70] |
khazanova1966 | [52] | roof1967 | [58] |
krichevskii1967 | [67] | sun2011 | [66] |
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rath, S., Gampe, U. & Jäger, A. A Numerical Algorithm for Calculating Critical Points and Its Application to Predictive Mixture Models and Binary CO\(_2\) Mixtures. Int J Thermophys 44, 170 (2023). https://doi.org/10.1007/s10765-023-03275-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-023-03275-2