Abstract
Densities of two methane-rich binary mixtures were measured in the homogeneous liquid and the supercritical region at temperatures between (100 and 160) K using a low-temperature single-sinker magnetic-suspension densimeter. For each mixture, four isotherms were studied over the pressure range from (0.3 to 10.8) MPa. Molar compositions of the gravimetrically prepared methane-rich binary mixtures were approximately 0.01 butane and 0.02 isopentane, respectively, with the balance being methane. The relative expanded combined uncertainty (k = 2) of the experimental densities was estimated to be in the range of (0.02 to 0.06) %. Due to a supercritical liquefaction procedure and the integration of a special VLE-cell, it was possible to measure densities in the homogeneous liquid phase without changing the composition of the liquefied mixture. Based on the supercritical liquefaction procedure, a new time-saving measurement procedure was developed and applied. Moreover, saturated-liquid densities were determined by extrapolation of the experimental single-phase liquid densities to the vapor pressure calculated with an equation of state (EOS); here, the relative expanded combined uncertainty (k = 2) is less than 0.05 % in most cases. The new experimental results were compared with the GERG-2008 equation of state, the EOS-LNG and the enhanced revised Klosek and McKinley (ERKM) method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The anthropogenic climate change driven by emissions of greenhouse gases (GHG) increases risks for natural and human systems [1, 2]. The European Union strives to reduce GHG emissions continuously and aims to achieve an economy with net-zero GHG emissions by 2050 [3]. Nevertheless, the global natural gas demand is expected to rise, and it is increasingly supplied as liquefied natural gas (LNG) [4]. Reaching higher efficiencies in all kinds of technological processes along the LNG value chain could, at least to a certain extent, reduce GHG emissions. The process efficiency improves significantly with the availability of accurate thermodynamic properties of LNG. Against this background, a series of Joint Research Projects as part of the European Metrology Research Programme (EMRP) and the European Metrology Programme for Innovation and Research (EMPIR) focusing on metrology for LNG, i.e., achieving smaller uncertainties for the quantities to be measured in custody transfer, were carried out.
Accurate determination of the energy quantity of the transferred LNG is essential in custody transfer; it depends (among other properties) on the density. State-of-the-art online density measurements would result in larger uncertainties in the calculated energy quantity compared to well-established equations of state (EOS) or correlation equations that are commonly used to calculate the density through online measurements of temperature, pressure and LNG composition as input variables. In our previous paper [5], we presented density data for seven binary methane-rich mixtures, including (methane + isobutane) and (methane + pentane) mixtures that were used for the development of a new fundamental equation of state for liquefied natural gases (EOS-LNG) by Thol et al. [6] in 2019. The EOS-LNG is based on the GERG-2008 equation of state by Kunz and Wagner [7], which was adopted as an ISO-standard for the calculation of thermodynamic properties of natural gas [8]. With new binary-specific departure functions developed for butanes and pentanes, the EOS-LNG could improve its prediction of densities for LNG containing these compounds compared to the GERG-2008 equation [6].
In the present paper, we report the results of accurate density measurements of two further selected methane-rich binary mixtures, one containing butane and one isopentane over the temperature range from (100 to 160) K at pressures up to 10.8 MPa. Such measurements of binary mixtures, in particular in the homogeneous liquid and the supercritical region, at low temperatures and over a wide pressure range are unique thus far. To the best of our knowledge, measurements like reported here have not been carried out elsewhere before as already discussed in our earlier work [5, 9,10,11,12]; other authors only measured data for saturated-liquid densities of binary mixtures and LNG-like mixtures [13,14,15,16,17,18,19,20,21] but not in the homogeneous liquid region below the cricondenbar pressure. For the presented two mixtures, however, no measurements of saturated-liquid densities could be found in literature. Hence, the new (p, ρ, T, x) data measured within this work can be used to assess the capability of the new EOS-LNG regarding these two binary systems and, moreover, can be used for further improvements of the model, if needed. The apparatus utilized for density measurements is a special single-sinker densimeter for cryogenic liquid mixtures [9,10,11].
2 Experimental Section
2.1 Apparatus Description
The density measurements reported in this paper were carried out with a precision densimeter, which was deliberately designed for accurate density measurements of cryogenic liquid mixtures such as LNG; it covers a temperature range from (90 to 300) K at pressures up to 12 MPa. Single-sinker densimetry, based on the Archimedes (buoyancy) principle, in conjunction with a magnetic-suspension coupling is utilized. The design of the cryogenic densimeter, the temperature and pressure measurement and the implementation of a special “VLE-cell” as a novel feature were described in detail previously [9,10,11]. In our latest paper [5], we presented some improvements of the core apparatus to reduce diffusion effects and the force-transmission error (FTE) [22, 23]. Here, for the convenience of the readers of the present data paper, we summarize the description of the apparatus presented by Richter et al. [11]. Overviews of this general type of densimeter were compiled by Wagner and Kleinrahm [24] as well as by McLinden [25].
The single-sinker method basically allows an absolute determination of the density of fluids. This method is applied in combination with a magnetic-suspension coupling and a load-compensation mechanism (differential method). A sinker of known volume \(V_{\text{S}} \left( {T,p} \right)\) and known mass \(m_{\text{S}}\) (in the present case: a single-crystal silicon, \(m_{\text{S}}\) ≈ 60.95 g, \(V_{\text{S}}\) ≈ 26.17 cm3, \(\rho_{\text{S}}\) ≈ 2.329 g·cm−3) is weighed in the fluid under study inside a pressure-tight measuring cell. Thus, the result of weighing the sinker immersed in the fluid, \(m_{{{\text{S}},{\text{fluid}}}}^{*}\), is the difference between the mass of the sinker and the buoyancy of the fluid:
where \(\rho_{\text{fluid}}\) is the density of the fluid. When weighing the sinker inside the evacuated measuring cell via the magnetic-suspension coupling, the weighing result is not the mass of the sinker, \(m_{\text{S}}\), but a slightly different result, \(m_{{{\text{S}},{\text{vac}}}}^{*}\), due to a small FTE of the magnetic-suspension coupling [22, 23]. Rearranging Eq. (1) yields the fluid density:
Equation (2) actually requires additional terms since essential details of the measurement procedure (e.g., the correction of the FTE due to the magnetic-suspension coupling) as discussed by Richter et al. (see Ref. [11], Appendix A) have to be taken in account. Moreover, the problem of the FTE due to the magnetic-suspension coupling is described in detail by Kleinrahm et al. [23].
To measure fluid density, the sinker is connected to an analytical balance (readability: 0.01 mg) employing an appropriate coupling/decoupling device. Gravity and buoyancy forces acting on the sinker are transmitted to the balance via the magnetic-suspension coupling, thus, isolating the fluid sample (which may be at high pressure and very low temperature) from the balance which is at ambient conditions. For compensation of the balance’s zero-point drift, the weighing procedure considers the small drift of the balance reading in the tare position by subtracting it from the balance reading in the measuring position. The balance is operated near its tare point using a load-compensation mechanism to reduce possible errors of the balance due to changes in the slope of the characteristic curve over the weighing range.
2.2 Experimental Material
The binary methane-rich gas mixtures were gravimetrically prepared and delivered in aluminum cylinders with an internal volume of 50 dm3 by Praxair Inc. The gas mixtures have been produced from high-purity starting materials, to minimize effects from impurities. The mole fractions of the principal components (methane and butane, and methane and isopentane, respectively) have been determined by comparison with the Dutch primary measurement standards in accordance with ISO 6143 [26] using gas chromatography. The configuration and measurement procedure have been described elsewhere [27]. This procedure establishes the metrological traceability of the measured mole fractions as well as the smallest uncertainties, both required to obtain credible reference data. Results of the analyses, which are traceable to primary and internationally accepted measurement standards, are listed in Table 1. The stated uncertainties in composition were reported by VSL in the calibration certificates as expanded uncertainties (k = 2), whereby the underlying standard uncertainties were determined according to the Guide to the expression of uncertainty in measurement (GUM) [28]. Further information on the mixtures is summarized in Tables 1 and 2. These are relevant for the experimental procedures described in Sect. 2.3.
To prevent any kind of change of the mixture composition (e.g., due to phase separation), we were very mindful regarding the sample handling. Filling the sample into the measuring cell of the densimeter with the correct composition was crucial. Hence, the sample cylinder was prepared according to the following procedure: (1) Rolling the sample cylinder for at least two hours. (2) Heating the cylinder at the bottom part for at least 3 h using a heating jacket to obtain vortices inside the sample cylinder for homogenizing the gas. (3) Filling the gas mixture into the well evacuated system to a pressure of about 0.2 MPa through the evacuated filling line and leaving the sample with a residence time of about 2 min before evacuating the apparatus again. Step (3) was repeated three times before the final filling. Thereby, residual components from previously studied samples are removed to prevent unwanted impurities of the new sample under study.
2.3 Experimental Procedures
Filling the densimeter and the basic procedure of running the apparatus were described exhaustively by Richter et al. [11]. This procedure was already used for our previous density measurements on synthetic LNG mixtures and methane-rich binary mixtures [5, 11, 12]. In the present work, this established procedure was applied for the density measurements on the (0.989 955 methane + 0.010 045 butane) mixture. For the density measurements on the (0.98 017 methane + 0.01 983 isopentane) mixture, however, a new procedure of running the apparatus after filling and cooling was developed and applied. Both procedures keep the sample composition as determined for the gas mixture in the sample cylinder before filling.
Measurements of the (0.98 017 methane + 0.01 983 isopentane) mixture showed an inexplicable increase (parallel offset) in the homogeneous liquid density at a certain step of the established basic method of Richter et al. [11]. It happened along all isotherms when the first vapor-liquid equilibrium (VLE) state point was set inside the VLE-cell (target value: approximately 20 % to 80 % liquid-volume fraction), and the increase in density was approximately (0.36 to 0.72) %, depending on temperature. This strange behavior was repeatable, and it is explained in more detail in the Electronic Supplementary Material (see Online Resource 1). Nevertheless, this issue was solved by applying a new procedure that does not involve the formation of a VLE inside the VLE-cell.
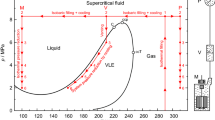
The new procedure is very similar to the basic procedure, by adopting a supercritical liquefaction method (filling above the cricondenbar pressure before cooling) in conjunction with a VLE-cell. The VLE-cell still serves as an essential buffer for the unavoidable phase transition caused by a VLE between the measuring cell at cryogenic conditions and the pressure measurement circuit that is thermostatted at a constant temperature of about 313.15 K (see Fig. 1). For comparison, the phase boundaries of the (0.98 017 methane + 0.01 983 isopentane) mixture were calculated with the GERG-2008 equation of state and the EOS-LNG equation of state and plotted in Fig. 1. The filling pressure was set conservatively above the cricondenbar calculated with the EOS-LNG.
Principle of the filling and liquefaction procedure shown for the example of the binary (0.98 017 methane + 0.01 983 isopentane) mixture in a p, T-diagram with subsequent density measurements along the 100 K isotherm.  , phase envelope calculated using the EOS-LNG equation of state by Thol et al. [6];
, phase envelope calculated using the EOS-LNG equation of state by Thol et al. [6];  , phase envelope calculated using the GERG-2008 equation of state of Kunz and Wagner [7, 8]; MC, measuring cell; P, pressure measurement circuit; C, critical point; ccp, cricondenbar; ccT, cricondentherm
, phase envelope calculated using the GERG-2008 equation of state of Kunz and Wagner [7, 8]; MC, measuring cell; P, pressure measurement circuit; C, critical point; ccp, cricondenbar; ccT, cricondentherm
However, the new procedure differs in two ways from the original one: (1) the pressure reductions to points (2 to 7) are not realized by venting sample. Instead, only the temperature of the VLE-cell is adjusted; (2) the VLE-cell remains in a homogenous liquid state even when the pressure is below the cricondenbar pressure and, therefore, is filled completely with homogenous liquid sample. The inevitable VLE will form somewhere between the VLE-cell and the pressure measurement circuit. Here, it is important to mention that the temperatures of the measuring cell and the VLE-cell can be controlled independently of each other at different set points and that all volumes of the measurement system are interconnected. Hence, the pressure is everywhere the same (apart from a pressure head correction).
As can be seen in Fig. 1, the VLE-cell is not being cooled to the same temperature as the measuring cell (MC) due to the fact that this cell is being used to control the pressure of the entire system. This control can be achieved because the apparatus becomes a closed system by closing the sample inlet as soon as the target temperatures are reached and, thus, the filling is completed. From this point on, the total mass of the sample in the system msys remains constant:
The internal volumes Vi of the components filled with fluid as well as the temperatures of measuring cell TMC and the pressure measurement circuit TP are virtually constant. Hence, due to the conservation of mass, a reduction of the VLE-cell temperature TVLE will cause a reduction of the system pressure p since the fluid density in the VLE-cell ρVLE will increase due to the decreasing temperature of the VLE-cell.
This interrelationship is utilized to sequentially reduce the pressure to approach the different state points along the isotherm of interest. Using appropriate equations of state for the fluid under investigation, the temperatures of the VLE-cell to reach certain pressures can be calculated in advance by using a self-written software tool. Because the difference between the isotherm T = 100 K and the temperature of the saturated-liquid line is quite large (see Fig. 1), venting of sample was not required for density measurements along the T = 100 K isotherm of the (0.98 017 methane + 0.01 983 isopentane) mixture at all. For the temperatures of (120, 140 and 160) K, however, the VLE-cell would need to be cooled to temperatures below the measuring cell temperature to reach the lowest pressures; but this has to be avoided to maintain the composition of the liquid mixture in the measuring cell. Hence, as a precautionary measure, the last 1, 2, and 3 state points at T = (120, 140, 160) K, respectively, were realized by venting sample from the system. For these state points the volume of the vapor phase in the VLE-cell was less than 20 %, except for the T = 120 K where it was approximately between (20 % and 50 %). To monitor the liquid level in the VLE-cell, a special liquid-level indicator was used, that is integrated in the VLE-cell; it enables the reliable determination of the liquid level in the VLE-cell (see Sect. 2.3 in Ref. [11]).
In most cases, the new procedure enables the operation of the apparatus for density measurements along an isotherm almost entirely via remote access to the lab computer. Only for the initial filling and cooling as well as for evacuation of the apparatus, presence in the laboratory is necessary. These changes in the procedure accelerated the measurements significantly. Furthermore, the total composition of the sample in the entire measurement system remains unchanged until eventual venting was required to reach certain state points. Moreover, since the VLE-cell is kept in the homogenous liquid region during almost the entire investigation, it serves as an even more effective buffer between the measuring cell and the phase transition (liquid to vapor).
2.4 Uncertainty in Density Measurement
The uncertainty in density measurement was determined in line with the GUM [28]. Assuming that there is no correlation of the input quantities, the expanded combined uncertainty \(U\) for the determination of cryogenic liquid densities using the above described densimeter can be determined by:
where \(u\left( \rho \right)\), \(u\left( T \right)\) and \(u\left( p \right)\) are the standard uncertainties in density, temperature and pressure, respectively; \(u\left( {\rho \left( x \right)} \right)\) corresponds to the standard uncertainty in the density resulting from the uncertainty of the gas composition; \(u\left( {\rho_{\text{repro}} } \right)\) accounts for an additional uncertainty from the reproducibility of our measurements; and \(u\left( {\rho_{\text{corr}} } \right)\) takes into account the uncertainty of the correction of the force-transmission error. Detailed description of the uncertainty analysis was given in previous works [5, 11, 12]. The expanded combined uncertainty in measurement was determined for each measured state point. Table 3 shows the contributions to the relative combined expanded uncertainty in density of an exemplary density measurement of the (0.98 018 methane + 0.01 982 isopentane) mixture at T = 100 K and p = 0.3018 MPa.
The main contribution (almost 89 %) to the combined standard uncertainty in density for the (0.98 017 methane + 0.01 983 isopentane) mixture is the uncertainty of the gas composition \(u\left( {\rho \left( x \right)} \right)\). Compared to results of methane-rich binary mixtures presented in a previous paper [5] and compared to the (0.989 955 methane + 0.010 045 butane) mixture, the (0.98 017 methane + 0.01 983 isopentane) mixture reveals the largest \(u\left( x \right)\) (see Table 1).
3 Results and Discussion
The densities of two selected methane-rich binary mixtures, one containing butane and one isopentane, were measured in the homogeneous liquid and the supercritical region. Furthermore, saturated-liquid densities were determined.
3.1 Results for Homogeneous Liquid and Supercritical Densities
For both mixtures, the densities were measured along the four isotherms T = (100, 120, 140 and 160) K; six to nine state points were investigated along each isotherm. Starting always with the supercritical filling pressure and ending close to the saturated-liquid pressure results in a pressure range from about (0.3 to 10.8) MPa. To avoid a change in composition for the liquefied samples, the filling of the apparatus as well as all measurements in the supercritical region were carried out at pressures at least 0.5 MPa above the cricondenbar pressure pccp (see Table 2). Furthermore, to avoid vaporization of the mixture in the measuring cell, all measurements in the liquid region were carried out at pressures at least 0.1 MPa above the vapor pressure psat. Two or three replicates were measured at each state point, and in total for both mixtures, density measurements were carried out at 27 to 29 (p, T) state points.
The experimental results of the (p, ρ, T, x) measurements of the (0.989 955 methane + 0.010 045 butane) and the (0.98 017 methane + 0.01 983 isopentane) mixture are listed in Table 4 together with the corresponding state point uncertainties as discussed in Sect. 2.4. The relative expanded combined uncertainty (k = 2) in density was estimated to be less than 0.020 % for the (0.989 955 methane + 0.010 045 butane) mixture, and for the (0.98 017 methane + 0.01 983 isopentane) it was estimated to be between (0.041 and 0.062) %. The difference between these two uncertainties is primarily caused by the uncertainty contribution resulting from the uncertainty of the gas composition \(u\left( {\rho \left( x \right)} \right)\), which is more than five times higher for the (0.98 017 methane + 0.01 983 isopentane) mixture (see Table 1).
The relative deviations of the experimental densities from values calculated with the GERG-2008 equation of state of Kunz and Wagner [7, 8] (as implemented in TREND 4.0 [35]) are also reported in Table 4. The GERG-2008 equation of state [7, 8] (as implemented in TREND 4.0 [35]) predicts an equilibrium of two liquid phases (LLE) for the (0.98 017 methane + 0.01 983 isopentane) mixture below the homogenous liquid phase at temperatures T ≈ (107.6 to 114.0) K at p = (0.3 to 11.0) MPa, respectively, where the phase boundary between these two points is approximately a straight line. However, due to the stability of our density measurements at T = 100 K and comparing flash calculations with the EOS-LNG [6] (as implemented in TREND 4.0 [35]) we are convinced that the temperatures of this phase boundary are below 100 K. NIST’s REFPROP v10.0 database [34] calculates with the GERG-2008 equation of state [7, 8] the density values in the homogenous liquid phase as REFPROP v10.0 does not address LLE (see Table 4, footnote c).
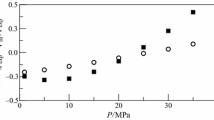
In Figs. 2 and 3, the relative deviations of the experimental densities from values calculated with the GERG-2008 equation of state are plotted versus pressure. The relative deviations for the (0.989 955 methane + 0.010 045 butane) mixture are less than 0.11 %. The experimental densities of the (0.98 017 methane + 0.01 983 isopentane) mixture deviate by approximately 2.2 % from values calculated with the GERG-2008 equation of state. The authors of the GERG-2008 equation report an uncertainty (k = 2) of (0.2 to 0.5) % for the binary (methane + butane) system and (0.5 to 1) % for the (methane + isopentane) system. These estimated uncertainties are valid for the liquid phase at pressures up to 40 MPa and T/\(T_{\text{r}}\) ≤ 0.7. For example, the reduced temperatures \(T_{\text{r}}\) for the two mixtures mentioned above are \(T_{\text{r}}\) = 193 K and \(T_{\text{r}}\) = 197 K, respectively. Hence, the GERG-2008 equation represents the experimental data of the (0.989 955 methane + 0.010 045 butane) mixture within the uncertainty reported for this equation. Nevertheless, the uncertainty of less than 0.02 % of the new experimental density values is much smaller. For the (0.980 17 methane + 0.01 983 isopentane) mixture, however, the relative deviations of approximately 2.2 % exceed the reported uncertainty of the equation of Kunz and Wagner [7, 8]. The reason for these larger deviations was the insufficient data situation in the liquid phase when the GERG-2008 equation was developed. The accuracy of this equation for both binary mixtures depends on its capability to describe the residual mixture behavior of these mixtures, which can be done in the most accurate way by so-called binary-specific departure functions. For both mixtures this was not possible because there were no experimental densities in the homogeneous liquid region available at that time. Against this background and the availability of new data [5], a new equation of state for LNG (EOS-LNG) was developed by Thol et al. [26] in 2019. The development of the new equation of state for liquefied natural gases was also carried out within the scope of a Joint Research Project within the European Metrology Programme for Innovation and Research (EMPIR).
Relative deviations of experimental and calculated densities ρ for the (0.989 955 methane + 0.010 045 butane) mixture from densities ρGERG calculated with the GERG-2008 equation of state [7, 8]. (p, ρ, T, x) data measured in the present work: ○, T = 100 K; ✕, T = 120 K; ☐, T = 140 K; +, T = 160 K. Densities calculated with the EOS-LNG [6]:  , T = 100 K;
, T = 100 K;  , T = 120 K;
, T = 120 K;  , T = 140 K;
, T = 140 K;  , T = 160 K. Densities calculated with the ERKM method [31]:
, T = 160 K. Densities calculated with the ERKM method [31]:  , T = 100 K;
, T = 100 K;  , T = 120 K
, T = 120 K
Relative deviations of experimental and calculated densities ρ for the (0.980 17 methane + 0.01 983 isopentane) mixture from densities ρGERG calculated with the GERG-2008 equation of state [7, 8]. (p, ρ, T, x) data measured in the present work: ○, T = 100 K; ✕, T = 120 K; ☐, T = 140 K; +, T = 160 K. Densities calculated with the EOS-LNG [6]:  , T = 100 K;
, T = 100 K;  , T = 120 K;
, T = 120 K;  , T = 140 K;
, T = 140 K;  , T = 160 K. Densities calculated with the ERKM method [31]:
, T = 160 K. Densities calculated with the ERKM method [31]:  , T = 100 K;
, T = 100 K;  , T = 120 K
, T = 120 K
The relative deviations of densities calculated with the EOS-LNG equation [6] (as implemented in TREND 4.0 [35]) from values calculated with the GERG-2008 equation [7, 8] are also shown in Figs. 2 and 3. It can be seen that the deviations of these calculated values from the new experimental densities of the (0.989 955 methane + 0.010 045 butane) mixture are less than 0.03 % in most cases, except for the 160 K isotherm, where the deviations increase up to 0.10 % at lower pressures. For the (0.98 017 methane + 0.01 983 isopentane) mixture, the EOS-LNG describes the new experimental values within 0.22 %. The authors reported, that their deviations from experimental densities were in general smaller than or at least similar to those of the GERG-2008 equation of Kunz and Wagner [7, 8]. Therefore, its uncertainty is ≤ (0.2 to 0.5) % for the binary (methane + butane) system and ≤ (0.5 to 1) % for the (methane + isopentane) system.
Moreover, we compared the new data for both mixtures with the enhanced revised Klosek and McKinley (ERKM) method by Tietz et al. [31] (as implemented in TREND 4.0 [35]). This method is based on a well-established model for the calculation of saturated-liquid densities within the field of LNG custody transfer, which is the revised Klosek and McKinley (RKM) method [32] of 1980. In Europe, for example, the RKM is recommended for the calculation of LNG densities by the GIIGNL LNG Custody Transfer Handbook [33]. The new ERKM method of 2017 also enables density calculations of LNG mixtures in the homogeneous liquid region in the temperatures range from (100 to 135) K at pressures up to 10 MPa. The estimated expanded uncertainty (k = 2) of the new method is reported by the authors to be 0.10 % for the temperature range from (100 to 115) K and 0.15 % for the temperature range from (115 to 135) K at pressures up to 10 MPa. The relative deviations of these calculated values from the new experimental densities are less than 0.08 % for both mixtures. However, we note that the ERKM method was developed for multicomponent mixtures and is not recommended for density calculation of binary mixtures.
3.2 Determination of Saturated-Liquid Densities
For both binary mixtures, saturated-liquid densities in the temperature range from (100 to 160) K were determined with the same method as in previous papers of our group [5, 11, 12]. The results are listed in Table 5. For their determination, the relative deviations of the experimental densities in the homogenous liquid phase from values calculated with the EOS-LNG equation of state (as implemented in TREND 4.0 [35]) were extrapolated to the mixture’s vapor pressure. The vapor pressures needed for the extrapolation of the densities along isotherms were computed with the EOS-LNG equation of state by Thol et al. [6] (as implemented in TREND 4.0) [35]. The authors reported, that their deviations from experimental vapor pressures were in general smaller than or at least similar to those of the GERG-2008 equation of Kunz and Wagner [7, 8]. Therefore, its uncertainty is ≤ (1 to 5) % (see Table 5, footnote a). The influence of the extrapolation on the uncertainty of the saturated-liquid density is relatively small in most cases, except for the highest temperature T = 160 K. The reason for the larger uncertainties (see Table 5) are larger isothermal compressibility (∂ρ/∂p)T near the phase boundary in combination with the uncertainty of the calculated vapor pressure.
In Fig. 4, the relative deviations of the experimental saturated-liquid densities of the two binary mixtures from values calculated with the GERG-2008 equation of state [7, 8] (as implemented in TREND 4.0 [35]) are plotted versus temperature. The values of these relative deviations and of the densities ρsat,exp, determined by extrapolation, are listed in Table 5.
Relative deviations of experimental and calculated saturated-liquid densities ρsat for two (methane + hydrocarbon) mixtures from densities ρsat,GERG calculated with the GERG-2008 equation of state [7, 8] (zero line). Diagrams: left (0.989 955 methane + 0.010 045 butane), right (0.980 17 methane + 0.01 983 isopentane). ○, (p, ρ, T, x) data of the present work;  , densities calculated with the EOS-LNG [6]. The uncertainties of our new saturated-liquid density data are listed in Table 5. Note that the scaling of the ordinate is different for the two diagrams
, densities calculated with the EOS-LNG [6]. The uncertainties of our new saturated-liquid density data are listed in Table 5. Note that the scaling of the ordinate is different for the two diagrams
The uncertainty of the saturated-liquid densities of the (0.989 955 methane + 0.010 045 butane) mixture calculated with the GERG-2008 equation of state [7, 8] was reported by the authors to be (0.2 to 0.5) % for 100 K ≤ T ≤ 140 K. Due to a missing departure function for the (methane + isopentane) system, the uncertainty of the saturated-liquid densities of the (0.980 17 methane + 0.01 983 isopentane) was reported by the authors of the GERG-2008 equation as (0.5 to 1) % for 100 K ≤ T ≤ 140 K. Hence, the GERG-2008 equation describes the new experimental saturated-liquid densities for the (0.989 955 methane + 0.010 045 butane) mixture within its uncertainty. For the (0.980 17 methane + 0.01 983 isopentane) mixture, however, the deviations are approximately 2.25 %. For both mixtures and temperatures T > 140 K, the uncertainty of the calculated saturated-liquid densities by the GERG-2008 equation is not specified by the authors.
For comparison, the behavior of the new equation of state of Thol et al. [6] (as implemented in TREND 4.0 [35]) is also plotted in Fig. 4. As in the case of the experimental densities in the homogeneous liquid region, the new equation of state of Thol et al. [6] predicts the presented experimental saturated-liquid densities better than the GERG-2008 equation of state, except for the experimental saturated-liquid density of the (0.989 955 methane + 0.010 045 butane) mixture at 160 K.
Moreover, the values calculated with the ERKM method [31] agree within 0.08 % with the new experimental data at T = (100 and 120) K which is in the validity range of the method. Thus, the ERKM method represents the new experimental saturated liquid densities within its uncertainty although, as mentioned in Sect. 3.1, the method is not recommended for binary mixtures.
4 Conclusion
Accurate density measurements of two methane-rich binary mixtures were carried out along isotherms from T = (100 to 160) K at pressures up to 10.8 MPa, utilizing a special single-sinker densimeter for cryogenic liquid mixtures. A set of 56 (p, ρ, T, x) data and 8 (ρsat, T, x) data were determined. The experimental results were compared with the GERG-2008 equation of state of Kunz and Wagner [7, 8], the EOS-LNG equation of state of Thol et al. [6] and the ERKM method of Tietz et al. [31]. The relative deviations of the experimental values from values calculated with the GERG equation are less than 0.11 % for the (0.989 955 methane + 0.010 045 butane) mixture, whereas the experimental densities of the (0.98 017 methane + 0.01 983 isopentane) mixture deviate by approximately 2.2 % from values calculated with the GERG-2008 equation of state in most cases.
Thus, the GERG-2008 equation is able to describe the experimental values of the (0.989 955 methane + 0.010 045 butane) mixture within the reported uncertainty of (0.2 to 1.0) % for the liquid phase, however, this does not apply to the (0.98 017 methane + 0.01 983 isopentane) mixture. The reason for the relative large deviations between the GERG-2008 equation and the measurements is discussed in Sect. 3.1. Thol et al. [6] developed a new equation of state for LNG on the basis of the GERG-2008 equation of Kunz and Wagner [7, 8]. This new fundamental equation of state describes the new experimental densities better than the GERG-2008, and in most cases within the experimental uncertainty. The EOS-LNG describes the experimental densities of the (0.989 955 methane + 0.010 045 butane) mixture in most cases within deviations of less than 0.03 %, and the experimental densities of the (0.98 017 methane + 0.01 983 isopentane) mixture where represented within 0.22 %.
An enhanced model for the calculation of the densities of liquefied natural gases (LNG) is the Enhanced Revised Klosek and McKinley (ERKM) method [31], which is valid in the temperature range from (100 to 135) K at pressures up to 10 MPa. A comparison of the new experimental densities with values calculated from the ERKM method reveals only small deviations in the homogenous liquid phase and along the saturated-liquid line within 0.08 %.
References
V. Masson-Delmotte, P. Zhai, H. O. Pörtner, D. Roberts, J. Skea, P. R. Shukla, A. Pirani, W. Moufouma-Okia, C. Péan, R. Pidcock, S. Connors, J. B. Matthews, Y. Chen, X. Zhou, M. I. Gomis, E. Lonnoy, T. Maycock, M. Tignor, T. Waterfield, IPCC, 2018: Summary for policymakers. In: Global warming of 1.5 °C. An IPCC Special Report on the impacts of global warming of 1.5 °C above pre-industrial levels and related global greenhouse gas emission pathways, in the context of strengthening the global. Geneva, Switzerland. (2018)
J. Cook, S. Van Der Linden, E. Maibach, S. Lewandowsky, The consensus handbook. (2018) https://doi.org/10.13021/G8MM6P
European Union, Long-term low greenhouse gas emission development strategies (LT-LEDS). (2020)
International Energy Agency, Gas market report 2019 executive summary—analysis and forecast to 2024. (2019)
R. Lentner, P. Eckmann, R. Kleinrahm, R. Span, M. Richter, Density measurements of seven methane-rich binary mixtures over the temperature range from (100 to 180) K at pressures up to 9.7 MPa. J. Chem. Thermodyn. 142, 106002 (2020). https://doi.org/10.1016/j.jct.2019.106002
M. Thol, M. Richter, E.F. May, E.W. Lemmon, R. Span, EOS-LNG: a fundamental equation of state for the calculation of thermodynamic properties of liquefied natural gases. J. Phys. Chem. Ref. Data 48, 1–36 (2019). https://doi.org/10.1063/1.5093800
O. Kunz, W. Wagner, The GERG-2008 wide-range equation of state for natural gases and other mixtures: an expansion of GERG-2004. J. Chem. Eng. Data 57, 3032–3091 (2012). https://doi.org/10.1021/je300655b
International Organization for Standardization (ISO), ISO 20765-2:2015(E), Natural gas, calculation of thermodynamic properties – part 2: single-phase properties (gas, liquid, and dense fluid) for extended ranges of application. Geneva. (2015)
M. Richter, R. Kleinrahm, R. Span, P. Schley, A new apparatus for the accurate measurement of LNG densities. GWF Int. 1, 66–69 (2010)
M. Richter, R. Kleinrahm, R. Span, P. Schley, A new apparatus for accurate measurements of the densities of liquefied natural gas (LNG). Int Gas Res Conf Proc. pp 2776–2790. (2011)
M. Richter, R. Kleinrahm, R. Lentner, R. Span, Development of a special single-sinker densimeter for cryogenic liquid mixtures and first results for a liquefied natural gas (LNG). J. Chem. Thermodyn. 93, 205–221 (2016). https://doi.org/10.1016/j.jct.2015.09.034
R. Lentner, M. Richter, R. Kleinrahm, R. Span, Density measurements of liquefied natural gas (LNG) over the temperature range from (105 to 135) K at pressures up to 8.9 MPa. J. Chem. Thermodyn. 112, 68–76 (2017). https://doi.org/10.1016/j.jct.2017.04.002
J. Klosek, C. McKinley, Densities of liquefied natural gas and of low molecular weight hydrocarbons. In: First International Conference on LNG 1968. Institute of Gas Technology. Chicago, (1968). p 15
J.B. Rodosevich, R.C. Miller, Experimental liquid mixture densities for testing and improving correlations for liquefied natural gas. AIChE J. 19, 729–735 (1973). https://doi.org/10.1002/aic.690190408
M.J. Hiza, W.M. Haynes, W.R. Parrish, Orthobaric liquid densities and excess volumes for binary mixtures of low molarmass alkanes and nitrogen between 105 and 140 K. J. Chem. Thermodyn. 9, 873–896 (1977). https://doi.org/10.1016/0021-9614(77)90173-2
R.C. Miller, M.J. Hiza, Experimental molar volumes for some LNG-related saturated liquid mixtures. Fluid Phase Equilib. 2, 49–57 (1978). https://doi.org/10.1016/0378-3812(78)80004-1
M.J. Hiza, W.M. Haynes, Orthobaric liquid densities and excess volumes for multicomponent mixtures of low molar-mass alkanes and nitrogen between 105 and 125 K. J. Chem. Thermodyn. 12, 1–10 (1980). https://doi.org/10.1016/0021-9614(80)90109-3
W. Haynes, Measurements of orthobaric-liquid densities of multicomponent mixtures of lng components (N2, CH4, C2H6, C3H8, CH3CH(CH3)CH3, C4H10, CH3CH(CH3)C2H5, and C5H12) between 110 and 130 K. J. Chem. Thermodyn. 14, 603–612 (1982). https://doi.org/10.1016/0021-9614(82)90077-5
W.M. Haynes, Orthobaric liquid densities and dielectric constants of (methane + 2-methyl-propane) and (methane + n-butane) at low temperatures. J. Chem. Thermodyn. 15, 903–911 (1983). https://doi.org/10.1016/0021-9614(83)90123-4
J.E. Orrit, Orthobaric liquid densities of natural-gas-component mixtures. Fluid Phase Equilib. 12, 253–281 (1983). https://doi.org/10.1016/0378-3812(83)80065-X
J. Janisch, G. Raabe, J. Köhler, Vapor–liquid equilibria and saturated liquid densities in binary mixtures of nitrogen, methane, and ethane and their correlation using the VTPR and PSRK GCEOS. J. Chem. Eng. Data 52, 1897–1903 (2007). https://doi.org/10.1021/je700210n
M.O. McLinden, R. Kleinrahm, W. Wagner, Force transmission errors in magnetic suspension densimeters. Int. J. Thermophys. 28, 429–448 (2007). https://doi.org/10.1007/s10765-007-0176-0
R. Kleinrahm, X. Yang, M.O. McLinden, M. Richter, Analysis of the systematic force-transmission error of the magnetic-suspension coupling in single-sinker densimeters and commercial gravimetric sorption analyzers. Adsorption 25, 717–735 (2019). https://doi.org/10.1007/s10450-019-00071-z
W. Wagner, R. Kleinrahm, Densimeters for very accurate density measurements of fluids over large ranges of temperature, pressure, and density. Metrologia 41, S24–S39 (2004). https://doi.org/10.1088/0026-1394/41/2/S03
M. O. McLinden, CHAPTER 2. Experimental TECHNIQUES 1: Direct methods. In: Volume properties. Royal Society of Chemistry, Cambridge, pp 73–99. (2014)
International Organization for Standardization (ISO), ISO 6143:2001—Gas analysis—Comparison methods for determining and checking the composition of calibration gas mixtures. (2001)
A.M.H. van der Veen, E.T. Zalewska, D.R. van Osselen, T.E. Fernández, C. Gómez, J. Beránek, R.J. Oudwater, D.C. Sobrinho, M.C. Brum, C.R. Augusto, J. Fükö, T. Büki, Z. Nagyné Szilági, P.J. Brewer, M.L. Downey, R.C. Brown, M. Valkova, Z. Durisova, K. Arrhenius, B. Magnusson, H. Yaghooby, T. Tarhan, E. Engin, L.A. Konopelko, T.A. Popova, M.N. Pir, O.V. Efremova, International comparison CCQM-K112 biogas. Metrologia 57, 08011 (2020). https://doi.org/10.1088/0026-1394/57/1A/08011
International Organization for Standardization (ISO), ISO/IEC Guide 98-3:2008, Uncertainty of measurement—part 3: guide to the expression of uncertainty in measurement. Geneva, (2008)
International Organization for Standardization (ISO), ISO 6974-1:2012, Natural gas—determination of composition and associated uncertainty by gas chromatography—part 1: General guidelines and calculation of composition. (2012)
International Organization for Standardization (ISO), ISO 6974-2:2012—natural gas—determination of composition and associated uncertainty by gas chromatography—part 2: uncertainty calculations. (2012)
C. Tietz, M. Richter, R. Kleinrahm, R. Span, Enhancement of the revised Klosek and McKinley method for density calculations of liquefied natural gas (LNG) over the temperature range from (100 to 135) K at pressures up to 10 MPa. Fuel Process. Technol. 165, 19–26 (2017). https://doi.org/10.1016/j.fuproc.2017.04.016
R. D. McCarty, Nat. Bur. Stand. (U.S.) Technical Note 1030 (1980)
LNG Custody Transfer Handbook, fifth ed. Groupe International des Importateurs de Gaz Naturel Liquéfié (GIIGNL), Paris, (2017)
E.W. Lemmon, I.H. Bell, M.L. Huber, M.O. McLinden, NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-REFPROP, Version 10.0. National Institute of Standards and Technology, Standard Reference Data Program, Gaithersburg, (2018)
R. Span, R. Beckmüller, T. Eckermann, S. Herrig, S. Hielscher, A. Jäger, E. Mickoleit, T. Neumann, S.M. Pohl, B. Semrau, M. Thol, TREND. Thermodynamic Reference and Engineering Data 4.0. Lehrstuhl für Thermodynamik, Ruhr-Universität Bochum, (2019)
Acknowledgments
We thank Professor Roland Span and Dr. Monika Thol of Ruhr University Bochum for helpful discussions regarding the present topic. Moreover, we are thankful to Santiago Castaños Benito for supporting the present project within the scope of his master thesis. This work was part of the Joint Research Project ‘‘Metrological support for LNG custody transfer and transport fuel applications” (Grant No. JRP: 16ENG09 LNG III) and was carried out as part of the European Metrology Programme for Innovation and Research (EMPIR), which was co-funded by the European Union’s Horizon 2020 Research and Innovation Programme and the EMPIR participating countries within the European Association of National Metrology Institutes (EURAMET).
Funding
Open Access funding provided by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Eckmann, P., von Preetzmann, N., Cavuoto, G. et al. Density Measurements of (0.99 Methane + 0.01 Butane) and (0.98 Methane + 0.02 Isopentane) over the Temperature Range from (100 to 160) K at Pressures up to 10.8 MPa. Int J Thermophys 41, 156 (2020). https://doi.org/10.1007/s10765-020-02728-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10765-020-02728-2