Abstract
Males may increase their fitness by providing care to offspring or to unrelated infants of female “friends” to maximise future mating. The potential for paternal care depends on paternity certainty, particularly in multi-male, multi-female groups with polygynandrous mating. In crested macaques (Macaca nigra), there seems to be high potential for paternity certainty and need for paternal protection. However, male-mother affiliation (or “friendships”), not paternity, predicts male-infant affiliation, questioning whether males can identify their offspring reliably. Using a Bayesian approach, we investigated male responses to infant screams (N = 2,637) emitted during agonistic interactions with males being the friend of the infant, the friend of the infants’ mother, and/or the father of the infant. Overall, male responses to infant screams were low. Bayesian estimates showed considerable uncertainty; hence, results should be interpreted cautiously. However, males were slightly more likely to react if the infant or its mother was a friend of the male or if the infant was his offspring. Additionally, higher-ranking males were slightly more likely to respond than lower-ranking ones, and screams from infants of lower-ranking females were more likely to be responded to. This might indicate that males assess paternity based on their rank and that they assess the need to intervene. Given the limitations of our study and the uncertainty surrounding our results, future studies are needed before we can draw solid conclusions for crested macaques. Overall, our results are in line with other studies suggesting that male primates provide care to related and unrelated infants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Among social mammals, males can improve their direct fitness by increasing the chances of survival of existing offspring and by producing more future offspring through enhanced mating success. However, there is huge variation within the animal kingdom to which extent males provide care for their offspring depending on the degree to which males benefit from such a strategy (Clutton-Brock, 1991; Fernandez-Duque et al., 2009; van Schaik & Paul, 1996).
In primates, as in other mammals, mothers invest far more in offspring than fathers do, due to pregnancy and lactation, making it easy for males to elude parental care. For primates, it seems that paternal care, defined as any behaviour a father directs to his offspring that improves the fitness of the offspring (Trivers, 1972), is manifested only in a few distantly related species (reviewed in Fernandez-Duque et al., 2009; see also Storey & Ziegler, 2016). Reasons for limited paternal care are most likely the associated costs in terms of time and energy allocation, including potentially missed opportunities to mate with a receptive female (Trivers, 1972). Furthermore, in the vast majority of primate species, especially those living in multi-male, multi-female groups, females mate with more than one male, which is hypothesized to confuse paternity (van Noordwijk & van Schaik, 2000). Thus, paternal care is generally less expected in polygynandrous species, unless males have reliable information on paternity: for example, via post-birth recognition by phenotype matching (reviewed in Widdig, 2007).
Nevertheless, empirical evidence shows that in some primate species, males do provide some form of care to infants or juveniles, although with varying degrees of investment. For example, paternal care has been demonstrated in yellow baboons (Papio cynocephalus) where sires systematically support their offspring during agonistic encounters against unrelated juveniles (Buchan et al., 2003). In rhesus macaques (Macaca mulatta), fathers are more likely to affiliate (approach, groom, and show other friendly behaviour) with their offspring than nonsires with unrelated juveniles (Langos et al., 2013). In chimpanzees (Pan troglodytes), fathers groom and play more often with their offspring than with unrelated infants (Murray et al., 2016). Furthermore, in chacma baboons (Papio ursinus), males associate more with their own offspring than with other juveniles, enabling the former to access richer food resources (Huchard et al., 2013). Males have even been observed to risk their own health for their offspring. In Hanuman langurs (Semnopithecus entellus), for example, males protect their offspring from infanticidal males (Borries et al., 1999).
Interestingly, in several primates, males seem not necessarily to focus their care to their own offspring only. Care for unrelated offspring (hereafter termed male care) has been observed in Barbary macaques (Macaca sylvanus) (Kuběnová et al., 2019; Ménard et al., 2001; Paul et al., 1996), chacma baboons (Moscovice et al., 2009, 2010), and mountain gorillas (Gorilla beringei beringei) (Rosenbaum et al., 2015). Although it is possible that care for unrelated offspring may result from misjudgement of paternity, it also has been suggested that such kind of care serves a function in increasing male fitness. The “mating effort hypothesis” (Smuts & Gubernick, 1992), also called the care-then-mate strategy (Ménard et al., 2001), postulates that males providing infant care achieve more frequent future mating opportunities with the infant’s mother compared with other males. To do so, these males often form long-term and strong social relationships with mothers, sometimes called “friendships”. Those friendships have been observed in baboons and macaques (Smuts, 1985; Moscovice et al., 2009, 2010; Ostner et al., 2013; but see Silk, 2002 for using the term friendship in nonhuman primates), with male friends not necessarily having sired the infant, but providing care for it. Surprisingly, in chacma baboons, those males still take high risks by protecting infants of their female friends from infanticide (Palombit et al., 2000), suggesting a potentially high pay-off in terms of reproductive benefits for the male. However, to date, there is little support for the mating effort hypothesis, and the existing evidence is partially contradictory depending on whether the male or female perspective is considered (Kuběnová et al., 2019). In particular, it remains unclear whether males that support unrelated infants just misjudge their kin relationship with the infant. More studies are needed to understand under which conditions males provide care for infants and whether the presence of male care (i.e., absence of paternal care) indicates that males cannot discriminate own from unrelated offspring.
Crested macaques (Macaca nigra) are an interesting species in this regard. They live in polygynandrous multi-male, multi-female groups with male dispersal and female philopatry (Duboscq et al., 2013). Males compete fiercely over dominance, resulting in a short alpha male tenure (mean of 12 months; Marty et al., 2017). Male dominance status has a strong impact on male reproductive output; high-ranking males sire more offspring than other males (Engelhardt et al., 2017; Higham et al., 2021; Neumann et al., 2022). Furthermore, a genetic study of three groups for more than 3 years revealed a high male reproductive skew: mean alpha paternity of 65% per year (Engelhardt et al., 2017; Neumann et al., 2022). The observed range in alpha paternity of 29–100%, however, suggests that factors other than dominance may determine male reproductive success. Those factors remain unknown, but one possibility is that males use the care-then-mate strategy.
Nevertheless, there are reasons to assume that paternal care is important in crested macaques, because infant mortality is quite high; nearly 20% of infants die or disappear within their first year of life (Engelhardt & Perwitasari-Farajallah, 2008). Recent takeovers of the alpha-male position by an immigrant male negatively affected infant survival (Kerhoas et al., 2014), hinting at the occurrence of male infanticide in this species. Given the vulnerability of infants and the fact that paternity is to a large extent determined by male dominance, this produces a high potential for paternity certainty and a need for paternal protection in crested macaques. Hence, we might expect that males can assess paternity and provide paternal care. Interestingly, a study investigating male-infant relationships in crested macaques found that affiliative dyads do not seem to occur at random; therefore, they were labelled as “specific male-infant dyads” (Kerhoas et al., 2016). In such dyads, infants initiated more affiliation towards males (63.5%) than vice versa, most infants (86%) had one male friend only, and infants had a five times higher probability of sitting near a male when their mother was absent. Moreover, the same study found that whereas males initiated affiliation more when the infant’s mother was around, infants initiated affiliation with males more often in the absence of their mothers. Hence, these data suggest that infants form relationships with males independent from the mother. Different from what we expected, however, mother-male affiliation, not paternity, explained the occurrence of male-infant affiliation. In other words, males affiliating with infants were friends of the infant’s mother but not necessarily the father of the offspring (Kerhoas et al., 2016). This is interesting given that high-ranking males were more likely to be involved in male-infant affiliation (Kerhoas et al., 2016), but although high-ranking males sire more offspring (Engelhardt et al., 2017), male-infant affiliation was not explained by paternity (Kerhoas et al., 2016). This raises the question of whether male crested macaques cannot discriminate their own offspring reliably or whether males affiliate with infants to increase their future mating opportunities with the mother.
The purpose of this study was to further investigate male investment in infants. While we previously limited our study to understand affiliation and shared spatial proximity in male-infant dyads, this study focuses on male-infant relationships in situations in which male support for the infant might be urgent and at the same time not necessarily risk-free for the male. Such a situation occurs, for example, if an infant screams during conflict with a third party. Such agonistic screams are common in many animals, including primates, e.g., spider monkeys (Ateles geoffroyi) (Ordóñez-Gómez et al., 2015), vervet monkeys (Chlorocebus pygerythrus) (Mercier et al., 2019), various macaques (Macaca spec.) (Gouzoules & Gouzoules, 2000), and eastern chimpanzees (Fedurek et al., 2015). One function of such screams is to recruit help from bystanders ("recruitment screams," Gouzoules et al., 1984). Whether bystanders will intervene in those agonistic encounters might depend on a cost–benefit calculation (Mercier et al., 2019). The response of males to infant screams has rarely been investigated, but males respond to infant screams reported in vervet monkeys (Hauser, 1986) and chacma baboons (Moscovice et al., 2009). Here, we explored which males responded to infant screams produced during agonistic interactions within the group. We investigated three sets of males and tested whether the responding male was: the father of the screaming infant; the friend of the infant’s mother; and/or was part of a specific male-infant dyad with the screaming infant, as defined by Kerhoas et al. (2016). We predicted that if male crested macaques were able to identify their offspring, males would be more likely to respond to screaming infants who are their genetic offspring. We also predicted that males would be more likely to respond to screaming infants of their female friends, independent of being the father, should males follow the care-then-mate strategy. Furthermore, we predicted that males would be more likely to respond to their infant friends (regardless of the relationship between the male and the mother of this infant) if they can neither identify their own offspring nor try to increase mating opportunities with female friends. Moreover, we investigated the effect of three other variables on the likelihood of male response: male dominance rank; maternal rank; and mother presence. Specifically, given the vulnerability of infants, for example in infanticidal attacks, infants would benefit from male protection (Kerhoas et al., 2014, 2016). Therefore, we predicted that high-ranking males are more likely to react to screaming infants than low-ranking males, as high-ranking males more likely have fathered (Engelhardt et al., 2017; Higham et al., 2021) and/or form affiliative bonds with infants (Kerhoas et al., 2016). Furthermore, infants of lower-ranking mothers are more likely to suffer higher vulnerability in agonistic interactions as they are less competitive and are therefore more in need of help than infants of higher-ranking mothers (Kerhoas et al., 2014). Consequently, we predicted that males are more likely to respond to infants of lower-ranking than higher-ranking mothers. Finally, if males follow the care-then-mate strategy, it is assumed that males provide care to infants to increase their future mating with the infant’s mother (Ménard et al., 2001). Accordingly, we predicted that mother presence will increase the likelihood of males to respond to the scream of infants.
Methods
Study Population
The study took place in the Tangkoko Nature Reserve in North Sulawesi, Indonesia (1°31′00.1″N, 125°10′59.9″E) characterized by primary and secondary (second-growth) lowland rain forest. We collected the behavioural data on three habituated groups of wild, crested macaques (R1, R2, and PB) that are regularly monitored by the Macaca Nigra Project (https://www.macaca-nigra.org/en/). Crested macaques are a Critically Endangered species (IUCN, 2020) endemic to the island of Sulawesi. Group sizes varied from 50 to 80 individuals during the study period (October 2008 to September 2010) with 13 to 25 reproducing females and four to 11 adult males in each group (see details Kerhoas et al., 2016). We recognized all adults and infants individually using physical characteristics.
Data Collection
DK and four field assistants collected 30-min, focal animal samples (Altmann, 1974), with good interobserver reliability (Cohen’s kappa = 0.67–0.80, significant correlation coefficients between behavioural variables = 0.74–0.96, all P < 0.05) (Kaufman & Rosenthal, 2009). We observed 35 infants (21 males, 14 females) in the three groups from birth to age 1 year (approximate weaning age; Kerhoas et al., 2014), in addition to five infants born before the start of data collection (i.e., already 1 to 6 months old). We collected behavioural data evenly throughout the day, from dawn to dusk, sometimes with several focal samples per day per infant. Thirty of 35 infants born survived the first year of their life (19 males, 11 females), and we collected 3,611 h of focal observations in total, with a mean of 100.63 h ± 23.87 (SD) per infant surviving to 1 year of age. Observation effort was consistent across all infants, with similar number of focal samples for each infant per month.
During each focal sample, we recorded the frequency of agonistic and affiliative interaction. For agonistic behaviour, we collected data on half open mouth threat, lunge, chase, and other agonistic interactions as described in Thierry et al. (2000). For affiliative behaviour, we noted 1) sociopositive approaches (i.e., an approach of at most 2.5 m for a minimum of 5 s that was not followed by immediate agonistic behaviour), 2) social grooming, and 3) friendly behaviour, such as lip smacks, silent bared teeth face, follows (i.e., an individual consistently walks after a moving partner) and peaceful interventions (i.e., intervening in conflict by directing affiliative behaviour towards one of the opponent) (all detailed in Kerhoas et al., 2016; and based on the ethogram described in Thierry et al., 2000). In total, we observed 24,082 male-infant affiliations in 3,611 observation hours, i.e., 6.67 affiliations per hour, > 90% of which were friendly approaches. The strength of male-infant association varied between dyads and infants initiated these affiliations twice as often as males (mean rate ± SD, infant affiliations towards males = 0.043 ± 0.054, male affiliations towards infants = 0.019 ± 0.016 (Kerhoas et al., 2016).
We also recorded all screams uttered by the focal infants when involved in intragroup conflicts during focal animal samples. These screams happened after the focal infant was involved in an agonistic interaction with another infant or juvenile (11.99%), an adult female (45.77%), or an adult male (42.24%) from the same group. In addition, we collected data on whether resident adult males reacted to a given infant scream or not. We defined a male response as 1) any friendly approach towards a screaming infant within 5 adult body lengths (or in 2.5-m radius) for a minimum of 5 s after an infant started screaming, and 2) any peaceful intervention by a male in an agonistic interaction between the focal infant and a third party by directing affiliative behaviour towards one of the opponents (as defined above). We did not include events in which males close to or approaching the infant close to a given scream showed agonistic behaviours towards the focal infant, given that we were only interested in males intervening or responding in an affiliative way to the screaming infant. Furthermore, we observed agonistic behaviors towards the screaming infant very rarely (fewer than 10 times across the study). To include only male responses caused by the respective scream under consideration, but also to allow a given male to approach from a distance, we counted male reactions within 3 min after onset of a scream. Following previous studies (Buchan et al., 2003), we assumed that all group members could hear infant screams, independent of their current distance between a given group male and an infant in need. In some screaming events, we observed that more than one male reacted which was considered in our analysis (see Results).
In addition, we recorded the frequency of all affiliative behaviour observed between the mother and an adult male when both were within 2.5 m of the focal infant as part of focal animal sampling. Furthermore, we recorded spatial proximity of the mother and adult males within a 2.5-m radius of the focal infant using scan sampling with 1-min intervals (Altmann, 1974). Finally, we collected ad libitum data (Altmann, 1974) on male migrations, displacement, or aggressive interactions to calculate group membership and male dominance hierarchies, respectively. To record data, we used Ptab software (Ptab Spreadsheet v.3.0; Z4Soft) on Hewlett Packard IPAQ Personal Digital Assistants (model 114) and Psion Workabout Pro M handhelds.
Variables Tested
We used most variables tested here already in our previous study, including male-infant friendship (or a male being an infants’ friend) (Kerhoas et al., 2016). In our previous study, we investigated factors that influenced male-infant affiliations and asked whether male-infants had specific bonds (or “specific male-infant bonds”), i.e., whether male-infant dyads displayed more affiliative interactions than expected at random. We performed permutation tests (Whitehead, 2008) on contingency tables containing the frequency of affiliative interactions (as defined above) initiated by infants towards males and vice versa, per study group. We permuted the identities of all the initiator of the interactions across the identities of the interactant. Furthermore, we used a chi-square test and applied 10,000 permutations, including the original data as one permutation. We determined the two-tailed P values as the proportion of chi-square values being at least as large as that of the original data. The results suggested that overall adult male-infant dyads did not affiliate at random, regardless of the group considered and whether infants or adult males initiated the interaction (Kerhoas et al., 2016). We scored dyads that had significantly higher observed frequencies of affiliation than the expected frequencies based on a binomial test as “specific male-infant dyads”. We chose a permutation approach because male-infant affiliation was not frequently observed, accepting that the permutation test cannot quantify the strength of the bond. Using this approach, we had found that 39 of all 187 possible dyads fall into the category of specific male-infant dyads. Infants maintained many of these specific dyads (48.7%, N = 19), whereas males maintained 15.4% (N = 6), with males and infants together maintaining 35.9% (N = 14). From the male perspective, 47% of males initiated bonds with only one infant, while 53% of males initiated bonds with two to four infants.
In the current analysis, we used the same specific male-infant dyads, but from the male perspective given that we were interested in male response. To align the different types of friendship used, we avoided the term “specific male-infant dyads” (Kerhoas et al., 2016) and named these dyads male-infant friends (or the male being the infants’ friend). In the analysis, we coded this variable for each male-infant dyad as a binary variable.
We assessed male-mother friendship (or a male being friend with the infants’ mother) by calculating the frequency of all affiliative behaviours (as defined above) exchanged between the mother and an adult male when both were within 2.5 m of an infant during the infant’s focal sample as in Kerhoas et al. (2016), controlling for observation time. Most affiliative behaviours collected were discrete (e.g., lip smacks); each event was counted and weighted the same. We counted a new grooming bout if there was a pause of more than 5 s. We used one male friend per female, which was the male with the highest frequency in affiliation towards a given female (hereafter “top friend”). We choose the top friend as the frequencies of male–female affiliations were highly skewed towards a single male, separating the most prominent male partner per female from other males (mean ± SD overall = 0.026 ± 0.038; top partner = 0.089 ± 0.063; second partner = 0.046 ± 0.026; third partner = 0.03 ± 0.018). This approach has the advantage of higher comparability between the male-infant and male-mother friendship variable, as both focused only on few dyads. Based on the observed differences in partner preference, it seems justifiable to select only the prominent partner. In the analysis, we coded this variable for each male as a binary variable based on whether he was the top friend of the infant’s mother or not.
We established male paternity (or a male being the infants’ father) based on genetic analysis (see below). Like the two previous predictors, we coded this as a binary variable for each male based on whether he was the father of a given infant or not.
We assessed male and female dominance separately via Elo ratings (Albers & de Vries, 2001; Neumann et al., 2011) based on same-sex displacements and aggressive dyadic interactions observed ad libitum (Altmann, 1974) as calculated in Kerhoas et al. (2016). In particular, for adult males, who are the dispersing sex (i.e., emigrating from and immigrating into groups), we used 6,784 interactions (agonistic encounters and displacements) between two males to establish dominance across the study period. We collected 3,721 interactions for adult females. We did not use a burn-in period to establish female and male dominance ranks, given that this species has regular dominance-related interactions, and we observed the groups almost every day during the study (Newton-Fisher, 2017). We gave each adult male and female a score of 1,000 (K value) at the start of data collection if they were present in the groups (or whenever a male immigrated in our study groups). This yielded an individual Elo rating score for each observational day for each adults, which accurately depicts dominance ranks in this species (Neumann et al., 2011). For analysis, we standardized these Elo scores to a range from 0 to 1 to obtain comparable ratings across the study. Because infants are expected to occupy a rank directly below their mother (Datta, 1988), we used maternal rank as a proxy of infant rank.
Finally, the variable mother presence in our current analysis reflects whether the mother was within five, adult, body lengths or 2.5 m during a given scream event of her infant. Again, we coded this variable as a binary variable.
Paternity Analysis
We collected noninvasive faecal samples from all focal infants (N = 30), their mothers (N = 30), and all potential sires (N = 41). We classified all adult males encountered in the study group before or during our study period as potential sires of all study infants. We excluded 11 natal subadult males as potential sires, because they reached adulthood sometimes after the infant was born. Because male age was unknown, we classified males with fully descended testes and fully erupted canines as adults (Kerhoas et al., 2014). We used a two-step method to store the samples after collection (Nsubuga et al., 2004). Specifically, we kept approximately 1 g of fresh faeces in 90% ethanol for 24 h, then moved it to a tube filled with 15 ml of silica until DNA extraction. After transfer to the laboratory, we extracted the samples with QIAamp DNA Stool Mini Kits (Qiagen) or GEN-IAL All-Tissue DNA Kits (GEN-IAL GmbH). In general, we genotyped a minimum of two independent faecal samples for all individuals (except for one infant with only one sample) to guard against sample mix-up and animal misidentification. We genotyped samples for 12 highly variable microsatellite markers (Engelhardt et al., 2017) and analysed products by using an ABI PRISM3100 automated sequencer and the ABI peak scanner software. We used a combination of the multiple tube approach (Taberlet & Luikart, 1999; Taberlet et al., 1996) and the two-step multiplex PCR (Arandjelovic et al., 2009) to increase the accuracy of the results (for PCR and genetic analysis details, see Kerhoas et al., 2016). We genotyped each individual at a minimum of eight loci, with a mean of 11.92 markers ± 0.31 (SD) genotyped per individual. For all infants studied, we genetically confirmed maternity derived from field observations and used this information subsequently in the paternity analysis.
For paternity assignment, we used a combination of exclusion and likelihood analyses. For 18 infants, we could exclude all potential sires on at least two loci, except for the assigned sire, who matched the mother–offspring pair at all investigated loci. For eight infants, we could exclude all potential sires on one locus, except for the assigned sire, who matched the mother–offspring pair. Furthermore, for three infants genotyped at all 12 loci, all potential sires mismatched the mother–offspring pair, but only one male had a single mismatch, whereas all other potential sires had at least two mismatches. We assigned the male with the single mismatch as the most likely sire. Finally, in one case, we excluded all potential sires on at least two loci, but two males had no mismatch. However, one of these two males was not in the group at the time the infant was conceived and immigrated a year later. Given the lack of evidence for extra-group paternity in our population so far (Engelhardt et al., 2017), we assigned the male who was present in the group at the time the infant was conceived as the most likely sire. We additionally supported all paternity assignments at the 95% confidence level by using the likelihood method calculated in CERVUS 3.0 (Kalinowski et al., 2007). The majority of fathers (86.7%) were still present when the offspring completed their first year of life (Kerhoas et al., 2016).
Statistical Analyses
To investigate whether infant screams elicit behavioural reactions from adult males, we used Bayesian Regression Models (BRM) with a Bernoulli response distribution and “logit link” function (Bürkner, 2017). For each infant scream, we extracted data for all adult males being resident in the infant’s group on the day that we observed the scream (N = 2,637 screams), i.e., who could potentially respond to a given infant scream. This resulted in 17,799 data points. As response, we used a variable containing per combination of scream and male either a zero (for those males who did not respond to the scream) or a one (for those males who approached and/or showed affiliation towards the screaming infant and/or intervened peacefully in the conflict). Our analysis considered single and multiple male responses to infant screams.
We included as fixed effects 1) whether the male was the friend of the infant (infants’ friend), 2) whether the male was a friend of the infant’s mother (mothers’ friend), and 3) whether the male was the father of the infant. Furthermore, we included 4) male rank, 5) maternal rank, and 6) maternal presence as additional test predictors in our analysis. Before running the model, we z-transformed the continuous covariates to a mean of zero and a standard deviation of one to enable direct comparisons between estimates (Schielzeth, 2010). Furthermore, we included a one-dimensional spline for the day number (days since the study began) to account for temporal autocorrelation in the data using the function “s” in the R package “brms” (Bürkner, 2017). As random effects, we included the identity of the male and the infant, the dyad ID, and the scream ID. We tried to include group and year as random effects, but the model did not converge. We also incorporated random slopes, including the correlation parameter between random intercept and random slope. We did this for all three fixed effects within infant and male identity (Barr et al., 2013; Schielzeth & Forstmeier, 2009).
We fitted the models in R (R Core Team, 2017) using the function “brm” in the R-package “brms” (version 2.5.0). This package runs 2,000 iterations by default over four MCMC chains, with a “warm-up” period of 1,000 iterations per chain, resulting in 8,000 usable, posterior samples (Bürkner, 2017). On visual inspection, the MCMC showed stationarity and convergence to a common target. All Rhat values were < 1.01, suggesting that different chains came to the same conclusion and there were no divergent transitions after warm-up (Gelman et al., 2014). Because we had no previous information, we used wide priors with a normal distribution, a mean of 0 and a standard deviation of 10 for the predictor variables. We checked for collinearity running a GLM with the function “VIF” from the package “car” but found no issues with VIF (no value > 1.3).
Ethical Note
Field research and sample permits were provided by the Ministry of Research and Technology of the Republic of Indonesia (RISTEK), the Indonesian Ministry of Forestry and the Department for the Conservation of Natural Resources (BKSDA) of North Sulawesi. We followed the International Primatological Society code of best practices for field primatology (MacKinnon et al., 2014).
Data Availability
The data set used to run the statistical analysis and an overview of males responding or not are provided in Supplements 1 and 2, respectively.
Results
Our data suggest that males generally responded to infant screams at low rates, with 355 male responses to 314 infant screams (11.91% of the 2,637 infant screams observed) (supplement 2 for a detailed overview). In 275 cases, one male responded to the screaming infant; in 37 cases, two males responded. In two further cases, three males responded to the screaming infant.
Overall, the results of our model showed considerable uncertainty in our parameter estimates (Figs. 1 and 2). Still, the model revealed that the probability of responding to an infant scream among our test predictors may show some patterns. First, males that were the friend of an infant showed a slightly higher probability of reacting to infant screams than nonfriends (Fig. 1, Estimate for the overall effect (mean of the posterior distribution) = 0.435, SE (standard error) = 0.410, CI (95% credible interval) = [− 0.372, 1.294], positive posterior support 87.48%). The predicted probability that a male reacted to an infant scream was 1.8% for males that were not friends of the infant and 2.7% for males that were friends of the infant, a 52.8% increase for friends compared with nonfriends (Fig. 2a, male-infant friends: Estimate = 0.027, SE = 0.011, CI (95%) = [0.011, 0.062], nonfriends: Estimate = 0.018, SE = 0.004, CI (95%) = [0.011, 0.026]).
Model estimates for all predictors of male responses to infant screams in crested macaques at Tangkoko Nature Reserve (Sulawesi) between 2008 and 2010. Plot shows the estimates (dots; mean of the posterior distribution) and the 65%, 85%, and 95% credible intervals (green lines) for all six predictors tested. The density of the posterior distribution is shown as curved line above the horizontal credibility intervals.
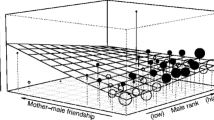
Probability of male affiliative responses towards infant screams in crested macaques at Tangkoko Nature Reserve (Sulawesi) between 2008 and 2010 as a function of six predictors: a) Friend of infant, b) Friend of mother, c) Sire, d) Male rank, e) Mother rank, and f) Mother presence. Coloured areas depict the 65%, 85%, and 95% credibility intervals of the mean predicted posterior distribution (horizontal line in Fig. 2a, b, c, f, and dashed line in Fig. 2d and e, respectively) for the probability of reaction. In plot 2a-c and 2f, each dot represents the mean probability of a given male; the dashed lines indicate the same male, in either of the categories shown. Overlapping data points are reflected by darker dots and lines. In plots 2d and 2e, the size of the circles indicates the sample size per value of the predictor. A rank of 0 indicates the lowest, a rank of 1 the highest rank per sex class.
Second, males who were the top friend of an infant’s mother also showed a slightly higher probability of responding to an infant’s scream than males who were not the top friend of the infant’s mother (Fig. 1, Estimate for the overall effect = 0.268, SE = 0.292, CI (95%) = [− 0.315, 0.802], positive posterior support 83.05%). The predicted probability that a male reacted to an infant scream was 1.8% for males who were not the top friend of the infant’s mother and 2.4% for males who were the top friend of the infant’s mother—a 30.5% increase for top friends compared with non-top friends (Fig. 2b, mother’s top friends: Estimate = 0.024, SE = 0.008, CI (95%) = [0.011, 0.044], mother’s non-top friends: Estimate = 0.018, SE = 0.004, CI (95%) = [0.011, 0.027]).
In addition, we found that sires were slightly more likely to react to infant screams than non-sires (Fig. 1, Estimate for the overall effect = 0.408, SE = 0.410, CI (95%) = [− 0.385, 1.248], positive posterior support 86.18%). The predicted probability that a male reacted to an infant scream was 1.6% for non-sires compared with 2.4% for sires, representing a 48.1% increase for sires compared to non-sires (Fig. 2c, sires: Estimate = 0.024, SE = 0.010, CI (95%) = [0.010, 0.056], non-sires: Estimate = 0.016, SE = 0.004, CI (95%) = [0.010, 0.025]).
The social status of males affected the likelihood of responding to an infant scream such that higher-ranking males were slightly more likely to respond than lower-ranking males (Fig. 1; Estimate = 0.121, SE = 0.144, CI (95%) = [− 0.163, 0.396], positive posterior support 80.04%). The predicted probability that a male reacted to an infant scream was 1.5% for the lowest-ranking males compared with 2.1% for the highest-ranking males—a 41.8% increase from the lowest to the highest male rank (Fig. 2d, lowest male rank: Estimate = 0.015, SE = 0.005, CI (95%) = [0.007, 0.027], highest male rank: Estimate = 0.021, SE = 0.006, CI (95%) = [0.011, 0.036]).
Males were more likely to respond to screams of infants of lower-ranking mothers than screams of infants of higher-ranking mothers (Fig. 1; Estimate = − 0.177, SE = 0.113, CI (95%) = [− 0.394, 0.048], negative posterior support 94.30%). The predicted probability that a male reacted to a scream of an infant of the highest-ranking females was 1.0% compared with 2.2% for the lowest-ranking females, an increase of more than twice (113.4%) from the highest to the lowest female rank (Fig. 2e, lowest female ranks: Estimate = 0.022, SE = 0.006, CI (95%) = [0.011, 0.037], highest female rank: Estimate = 0.010, SE = 0.004, CI (95%) = [0.005, 0.020]).
Finally, the presence of the mother at the conflict when the infant screamed did not have any notable effect on male responses (Fig. 1; Estimate = 0.082, SE = 0.167, CI (95%) = [− 0.249, 0.406], positive posterior support 69.45%). The predicted probability that a male reacted to a scream of an infant suggested no difference if the infant’s mother was present compared to if the infant’s mother was absent (Fig. 2f, mother present: Estimate = 0.016, SE = 0.004, CI (95%) = [0.009, 0.026], mother absent: Estimate = 0.015, SE = 0.003, CI (95%) = [0.009, 0.023]).
Discussion
Overall, our results show that male crested macaques respond rarely (11%) to screams of infants involved in agonistic interaction. Bayesian estimates showed considerable uncertainty, so our results should be interpreted cautiously. However, there may be some differences in responses between friends versus nonfriends and sires versus non-sires. In more detail, our data may suggest that males who were friends with the infant were slightly more likely to respond to infant screams than males that were not a friend of the infant. We also found that males who were the top friend of the infant’s mother were slightly more likely to respond to an infant’s scream than males who were not the top friends of the mother. Furthermore, the probabilities of male responses were slightly higher for fathers than nonfathers. These results add to our previous observations (Kerhoas et al., 2016) that male crested macaques either cannot assess paternity reliably or that paternal care is less important than would be expected in this species. Furthermore, higher-ranking males were slightly more likely to respond than lower-ranking ones, suggesting that males may base paternity assessment on their rank. Males also were more likely to support infants of lower-ranking females, i.e., more vulnerable infants, suggesting that males assess the need for an intervention. Finally, the presence of the mother at the conflict did not affect the likelihood of male response. Considering the Bayesian way of interpreting results by including the entire distribution rather than a point estimate, we overall conclude that the positive posterior support of 86% for fathers, 87% for infant friends, and 83% for mother’s top friend suggests a positive effect of paternity and friendship, respectively, on male responses to infant screams. However, given our parameter estimates, there is still a considerable chance for a negative relationship.
In crested macaques, paternity may have some effect on male response to infant screams suggesting some, although highly restricted, degree of paternal care in this species. This would corroborate findings of other studies focusing on paternal care in multi-male, multi-female polygynandrous species, showing that males affiliate with offspring or tolerate them at feeding sites, but rarely protect their offspring (Buchan et al., 2003; Huchard et al., 2013; Langos et al., 2013; MacKinnon et al., 2014; Minge et al., 2016; Murray et al., 2016). One study on infant protection by males stands out here, as male baboons selectively support their offspring when involved in agonistic disputes with other juveniles (Buchan et al., 2003) implying low-risk and low-cost investment by fathers (Geary, 2000; Langos et al., 2015). In rhesus macaques, males affiliate more with their offspring than males with unrelated juveniles (Langos et al., 2013), but males hardly ever intervene in favour of their offspring when they are involved in agonistic interactions (Kulik et al., 2012). Interestingly, in our previous study on male-infant affiliations in crested macaques, we found no effect of paternity on the occurrence of male-infant affiliations (Kerhoas et al., 2016). This might mean that any form of paternal care, if confirmed in this species, is subtle and constrained to certain situations, but expected to have evolved given the high degree of infant mortality in crested macaques (Engelhardt & Perwitasari-Farajallah, 2008). Our current analysis may suggest that crested macaque males potentially have some cues available to assess which infants they have sired. Still, males seem to invest in their offspring very rarely and only under certain circumstances. For example, our data revealed that infants screaming for help were involved in agonistic interactions mainly with adult males (42%) or females (46%) from the same group. Hence, males may have assessed their risk of intervening to avoid potential conflicts with male rivals. It might not be surprising that paternity shows a positive, although very limited, effect on offspring support when they are in need, but not when looking at male-infant affiliation only (Kerhoas et al., 2016).
There might be other reasons for why paternal care is not pronounced in crested macaques, and why we found no clear father-offspring affiliation (Kerhoas et al., 2016), but rare peaceful interventions in favour of their offspring (this study). The simplest explanation may be that crested macaque infants do not benefit from mere affiliation with their father (and vice versa) so that neither fathers nor infants invest into such relationships on a day-to-day basis. This possibility is supported by our previous study (Kerhoas et al., 2016), which found no influence of paternity on male-infant affiliation. However, both studies looked at infants up to 12 months only. A second alternative explanation might be that, although it would be potentially beneficial, fathers may not have the time to associate frequently with their offspring. Although there is quite some variation between years and groups with regard to male reproductive skew (i.e., the degree to which paternity is skewed towards a single or a few males), it is relatively high in crested macaques in general (Engelhardt et al., 2017). In one group during our study, the alpha male sired 100% of infants in 1 year (i.e., 15 infants in this study). However, only seven mothers and four infants were friends with this alpha male. Similarly, among all groups, nine of 30 infants and 15 of 30 females had an alpha male as friend. Thus, the pronounced skew is likely to prevent sires from staying close to all of their offspring. Another factor limiting male time to interact with offspring is the short alpha tenure in crested macaques. With a mean alpha tenure of 12 months (Marty et al., 2017), males should invest all this time in mating with a maximum number of fertile females. Indeed, with low synchrony in female fertile phases, dominant, male, crested macaques monopolize a high proportion of mating, resulting in a high paternity skew (Higham et al., 2021). Our data therefore may support the idea that males do not have time to invest in building affiliative bonds with their offspring, as males restrict their care to some affiliative support when infants are involved in agonistic interactions. Still, such support would critically affect infant survival and hence impact male fitness. Hence, it makes sense for fathers to focus their care to situations in which their support is urgent and localisation of offspring may be relatively easy through infant vocalisation.
A third reason that males may not interact very frequently with their offspring might derive from the fact that infanticide may occur in crested macaques. We previously showed that infant survival was impaired most after recent takeovers of the alpha-male position by an immigrant male (Kerhoas et al., 2014), with immigrants likely benefiting from infanticidal attacks in groups where they have not yet mated with any females. In our studies of male-infant relationships and in this study, we focussed on infants below the age of 1 year, i.e., those still dependent on their mothers and usually targeted by infanticidal males (Palombit et al., 2000). It therefore should be in the interest of both mothers and fathers to prevent their offspring from being killed; hence, our observations that fathers were slightly more likely than nonfathers to respond to screams of infants during their first year of life make sense. The active involvement of fathers in the prevention of infanticide has rarely been studied in primates but has been observed in Hanuman langurs (Borries et al., 1999) and chacma baboons (Palombit et al., 2000). However, infanticides are rare events among primates and therefore are difficult to observe in the wild (Dixson, 2013), making infanticide prevention difficult to study systematically. An alternative prevention of infanticide that females are hypothesized to employ strategically is mating with many males to confuse paternity, because males should refrain from killing potential offspring (Borries et al., 1999). Paternity certainty or confusion, however, may not be straight forward, but rather a question of probability (van Schaik, 2000). Males may assess the likelihood of paternity based on available cues, such as how often they have mated with a female at which state of her reproductive cycle or via post-birth recognition by phenotype matching (Widdig, 2007). Different males thus may have different assessments of the paternity of a given infant, some being correct while others are not. Hence, fathers may refrain from indicating their paternity in day-to-day affiliation with their offspring, but do so when supporting offspring on the few occasions when help is potentially more urgent or less risky to themselves.
Despite some indications that male crested macaques may have the ability to assess paternity likelihood, we also observed that being a friend of the infant or the infant’s mother slightly increased the likelihood of males to respond to infant screams compared to nonfriends. It might be that males responded to these infant screams merely because they falsely assumed they were the father. However, if this was not the case, our result may be some indication that male crested macaques follow the care-then-mate strategy. So far, little evidence supports this hypothesis. Male–female affiliation often has been seen as a female counterstrategy to infanticide (Palombit, 2009) by increasing the likelihood of male protection to the female (Lemasson et al., 2008) and possibly her infant (Nguyen et al., 2009). Whether infant care by unrelated males is indeed an active male strategy or a result of male misjudgement of paternity is still unclear. Because we do not have any mating data for when the infants were conceived, we are not able to assess this either. It may be that male friends had increased access to the mother during conception, because male friends are generally of high dominance rank in crested macaques (Kerhoas et al., 2016); however, high-ranking males may not always have fertilized their female friends, given that other factors than dominance sometimes determine paternity success (Engelhardt et al., 2017). In chacma baboons, where male friends protect females and their infants, females form friendships most often, but not always, with the father of their offspring (Moscovice et al., 2010). If the father is not present at the time of parturition, females form those bonds with other high-ranking males, who still hold a high likelihood of paternity (ibid). In both chacma baboons and crested macaques, males may perform infant care and protection as a form of paternal care based on imperfect assessment of paternity rather than as an opportunity to increase future mating opportunities.
A potential hint in this direction is probably given by one of our additional test variables: mother’s presence. When infants screamed during agonistic conflicts, the mother’s presence did not predict whether a male responded to an infant’s scream. However, to “impress” female friends and make them more likely to mate with male friends in the future, male friends should show infant care when the mother is around (similar to male friends affiliating more with infant when mothers are present, reported in Kerhoas et al., 2016). The fact that we did not find an effect of mother presence may show that it was more important for the male to support the infants, regardless of whether the mother was present. From our current data, we do not know whether males were aware of the mothers’ presence when they responded to the screaming infant. In contrast, fathers would be expected to help their own offspring regardless of the presence of mothers (similar to patterns of father-offspring affiliation, reported in Langos et al., 2013).
Finally, two other test variables seem to indicate that male crested macaques intend to carry out paternal care rather than increase their future mating chance. First, higher-ranking males, thus more likely fathers, were slightly more likely to respond to infant screams than lower-ranking ones. At the same time, in a species with high male-male competition, such as the crested macaques, those males could be expected to have less need to impress a female to increase their future reproductive opportunities. We would expect low-ranking males to invest more in affiliative bonds with mothers or in infant protection, but their risk of intervening when other males are involved in the agonistic interaction with the infant is probably high. An alternative explanation for males reacting and approaching towards conflicts involving a screaming infants could be policing behaviour. Macaque males, especially high-ranking males, are known for their policing (defined as impartial interventions functioning to control conflicts or to increase group stability; Beisner & McCowan, 2013; Flack et al., 2005; Petit & Thierry, 1994), as opposed to males supporting their offspring, their infant friend, or the infant of their female friend. Regardless of the driving force, the outcome in all cases would be the protection of infants. Second, our analysis suggested an effect of maternal rank; infants of lower-ranking females were more likely to receive male responses after a scream than those of higher-ranking females. Usually, higher-ranking females are expected to be the more attractive reproductive partners (Keddy-Hector, 1992). Therefore, one would expect males to try to bond with these females more often than with lower-ranking females. However, infants of lower-ranking females may be more vulnerable (Cheney & Seyfarth, 1987; Kerhoas et al., 2014; Pusey et al., 1997), so may need male support more urgently than other infants. Thus, males who are the most likely father of the infant should support them.
Our study design included several limitations. First, we lack a control for the opportunity for the male to react; hence, we cannot rule out the possibility that spatial patterns could explain our results. We collected data similar to those in Buchan et al. (2003), who investigated male interventions in agonistic disputes among juvenile baboons. Likewise, we collected data under the assumption that all group males, no matter whether peripheral or central to the core group (Neumann et al., 2013), could hear the infant screams and had the choice of responding or not. If this assumption is incorrect, we have likely inflated the number of coding 0 (no response) in cases in which some peripheral males may not have hear the infant screaming. However, the number of males per group in our study only varied from four to 11, so the potential bias may be limited. In some cases, more than one male responded to a given scream, and we controlled for this in our analysis to allow distant males to approach to the conflict. Future studies should collect focal data from the male (not infant) perspective and report who is around when the infant screamed and who responded to the scream.
Second, our data on male–female affiliation were collected as part of the infant focal sample; hence, we recorded male–female affiliation only when they were near the infant. This is because we collected our data primarily to study male-infant associations (Kerhoas et al., 2016) and used these data as a follow-up study to examine whether males respond to infants when they are potentially in need. Although these data are not ideal, they still revealed a five times higher probability of an infant being near to a male in the absence of its mother and a six times higher probability of the infant being with its mother and without the male, which stresses that infants can form relationships with males independent from the mother (Kerhoas et al., 2016). Finally, to reduce complexity in infant-male and male–female affiliations, respectively, we used the top male friend only, as male-mother affiliation was highly skewed towards one top partner. This choice was in line with the fact that there is only one father per infant and one infant friend for most males. Together, these decisions might have influenced the outcome of our model. Hence collecting extended data without these limitations would be needed before we can draw solid conclusions about whether male crested macaques can correctly assess paternity and why other males may invest in unrelated infants, for example because males misjudge paternity. Specifically, more studies are needed of male–female reproductive interactions before an infant is born, and after it has been weaned, to clarify whether male–female affiliations are a male strategy to increase male future reproductive opportunities in crested macaques. Future research on this topic may involve acoustic playback experiments to disentangle the effects of paternity and friendship in more detail. Such an experimental setup could control for the potential of male time constraints by presenting infant screams 1) when fathers and/or male friends are present to react to infant screams or not, 2) when males may have time conflict to react to infant screams or not, and 3) when mothers are present or not.
References
Albers, P., & de Vries, H. (2001). Elo-rating as a tool in the sequential estimation of dominance strengths. Animal Behaviour, 61(2), 489–495. https://doi.org/10.1006/anbe.2000.1571
Altmann, J. (1974). Observational study of behavior: Sampling methods. Behaviour, 49, 227–267. https://doi.org/10.1163/156853974X00534
Arandjelovic, M., Guschanski, K., Schubert, G., Harris, T. R., Thalmann, O., Siedel, H., & Vigilant, L. (2009). Two-step multiplex polymerase chain reaction improves the speed and accuracy of genotyping using DNA from noninvasive and museum samples. Molecular Ecology Resources, 9(1), 28–36. https://doi.org/10.1111/j.1755-0998.2008.02387.x
Barr, D. J., Levy, R., Scheepers, C., & Tily, H. J. (2013). Random effects structure for confirmatory hypothesis testing: Keep it maximal. Journal of Memory and Language, 68(3), 255–278. https://doi.org/10.1016/j.jml.2012.11.001
Beisner, B. A., & McCowan, B. (2013). Policing in Nonhuman Primates: Partial Interventions Serve a Prosocial Conflict Management Function in Rhesus Macaques. PLoS ONE, 8(10), 1–13. https://doi.org/10.1371/journal.pone.0077369
Borries, C., Launhardt, K., Epplen, C., Epplen, J. T., & Winkler, P. (1999). Males as infant protectors in Hanuman langurs (Presbytis entellus) living in multimale groups- defence pattern, paternity and sexual behaviour. Behavioral Ecology and Sociobiology, 46, 350–356.
Buchan, J. C., Alberts, S. C., Silk, J. B., & Altmann, J. (2003). True paternal care in a multi-male primate society. Nature, 425(6954), 179–181. https://doi.org/10.1038/Nature01866
Bürkner, P.-C. (2017). brms: An R Package for Bayesian Multilevel Models Using Stan. Journal of Statistical Software, 80(1), Article 1. https://doi.org/10.18637/jss.v080.i01
Cheney, D. L., & Seyfarth, R. M. (1987). The influence of intergroup competition on the survival and reproduction of female vervet monkeys. Behavioral Ecology and Sociobiology, 21(6), 375–386.
Clutton-Brock, T. H. (1991). The evolution of parental care. Princeton University Press.
Datta, S. (1988). The acquisition of dominance among free-ranging rhesus monkey siblings. Animal Behaviour, 36(3), 754–772. https://doi.org/10.1016/S0003-3472(88)80159-3
Dixson, A. F. (2013). Male infanticide and primate monogamy. Proceedings of the National Academy of Sciences, 110(51), E4937–E4937. https://doi.org/10.1073/pnas.1318645110
Duboscq, J., Micheletta, J., Agil, M., Hodges, K., Thierry, B., & Engelhardt, A. (2013). Social Tolerance in Wild Female Crested Macaques (Macaca nigra) in Tangkoko-Batuangus Nature Reserve, Sulawesi, Indonesia. American Journal of Primatology, 75(4), 361–375. https://doi.org/10.1002/ajp.22114
Engelhardt, A., Muniz, L., Perwitasari-Farajallah, D., & Widdig, A. (2017). Highly polymorphic microsatellite markers for the assessment of male reproductive skew and genetic variation in critically endangered crested macaques (Macaca nigra). International Journal of Primatology, 38(4), 672–691. https://doi.org/10.1007/s10764-017-9973-x
Engelhardt, A., & Perwitasari-Farajallah, D. (2008). Reproductive biology of Sulawesi crested black macaques (Macaca nigra). Folia Primatologica, 79, 326.
Fedurek, P., Slocombe, K. E., & Zuberbühler, K. (2015). Chimpanzees communicate to two different audiences during aggressive interactions. Animal Behaviour, 110, 21–28. https://doi.org/10.1016/j.anbehav.2015.09.010
Fernandez-Duque, E., Valeggia, C. R., & Mendoza, S. P. (2009). The biology of paternal care in human and nonhuman primates. Annual Review of Anthropology, 38, 115–130. https://doi.org/10.1146/annurev-anthro-091908-164334
Flack, J. C., de Waal, F. B. M., & Krakauer, D. C. (2005). Social Structure, Robustness, and Policing Cost in a Cognitively Sophisticated Species. The American Naturalist, 165(5), E126–E139. https://doi.org/10.1086/429277
Geary, D. C. (2000). Evolution and proximate expression of human paternal investment. Psychological Bulletin, 126(1), 55–77. https://doi.org/10.1037/0033-2909.126.1.55
Gelman, A., Carlin, J. B., Stern, H. S., Dunson, D. B., Vehtari, A., & Rubin, D. B. (2014). Bayesian data analysis (Third Edition). CRC Press.
Gouzoules, H., & Gouzoules, S. (2000). Agonistic screams differ among four species of macaques: The significance of motivation-structural rules. Animal Behaviour, 59, 501–512.
Gouzoules, S., Gouzoules, H., & Marler, P. (1984). Rhesus monkey (Macaca mulatta) screams: Representational signalling in the recruitment of agonistic aid. Animal Behaviour, 32, 182–193.
Hauser, M. D. (1986). Male responsiveness to infant distress calls in free-ranging vervet monkeys. Behavioral Ecology and Sociobiology, 19, 65–71.
Higham, J. P., Heistermann, M., Agil, M., Perwitasari-Farajallah, D., Widdig, A., & Engelhardt, A. (2021). Female fertile phase synchrony, and male mating and reproductive skew, in the crested macaque. Scientific Reports, 11(1), Article 1. https://doi.org/10.1038/s41598-021-81163-1
Huchard, E., Charpentier, M. J., Marshall, H., King, A. J., Knapp, L. A., & Cowlishaw, G. (2013). Paternal effects on access to resources in a promiscuous primate society. Behavioral Ecology, 24(1), 229–236. https://doi.org/10.1093/beheco/ars158
IUCN. (2020). The IUCN Red List of Threatened Species. https://www.iucnredlist.org/en. Accessed 19 July 2020
Kalinowski, S. T., Taper, M. L., & Marshall, T. C. (2007). Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Molecular Ecology, 16(5), 1099–1106. https://doi.org/10.1111/j.1365-294X.2007.03089.x
Kaufman, A. B., & Rosenthal, R. (2009). Can you believe my eyes? The importance of interobserver reliability statistics in observations of animal behaviour. Animal Behaviour, 78(6), 1487–1491. https://doi.org/10.1016/j.anbehav.2009.09.014
Keddy-Hector, A. C. (1992). Mate choice in non-human primates. American Zoologist, 32, 62–70. https://doi.org/10.1093/icb/32.1.62
Kerhoas, D., Kulik, L., Perwitasari-Farajallah, D., Agil, M., Engelhardt, A., & Widdig, A. (2016). Mother-male bond, but not paternity, influences male-infant affiliation in wild crested macaques. Behavioral Ecology and Sociobiology, 70(9), 1117–1130. https://doi.org/10.1007/s00265-016-2116-0
Kerhoas, D., Perwitasari-Farajallah, D., Agil, M., Widdig, A., & Engelhardt, A. (2014). Social and ecological factors influencing offspring survival in wild macaques. Behavioral Ecology, 25(5), 1164–1172. https://doi.org/10.1093/beheco/aru099
Kuběnová, B., Ostner, J., Schülke, O., Majolo, B., Šmilauer, P., Waterman, J., Tkaczynski, P., & Konečná, M. (2019). The male and female perspective in the link between male infant care and mating behaviour in Barbary macaques. Ethology. https://doi.org/10.1111/eth.12948
Kulik, L., Muniz, L., Mundry, R., & Widdig, A. (2012). Patterns of interventions and the effect of coalitions and sociality on male fitness. Molecular Ecology, 21(3), 699–714. https://doi.org/10.1111/j.1365-294X.2011.05250.x
Langos, D., Kulik, L., Mundry, R., & Widdig, A. (2013). The impact of paternity on male–infant association in a primate with low paternity certainty. Molecular Ecology, 22(13), 3638–3651. https://doi.org/10.1111/mec.12328
Langos, D., Kulik, L., Ruiz-Lambides, A., & Widdig, A. (2015). Does male care, provided to immature individuals, influence immature fitness in Rhesus Macaques? PLoS ONE, 10(9), e0137841. https://doi.org/10.1371/journal.pone.0137841
Lemasson, A., Palombit, R., & Jubin, R. (2008). Friendships between males and lactating females in a free-ranging group of olive baboons (Papio hamadryas anubis): Evidence from playback experiments. Behavioral Ecology and Sociobiology, 62(6), 1027–1035.
MacKinnon, K., Riley, E., Garber, P., Setchell, J., & Fernandez-Duque, E. (2014). Code of Best Practices for Field Primatology. https://doi.org/10.13140/2.1.2889.1847, https://internationalprimatologicalsociety.org/wp-content/uploads/2021/10/Code-of_Best_Practices-Oct-2014.pdf
Marty, P. R., Hodges, K., Agil, M., & Engelhardt, A. (2017). Alpha male replacements and delayed dispersal in crested macaques (Macaca nigra). American Journal of Primatology, 79(7), e22448. https://doi.org/10.1002/ajp.22448
Ménard, N., Segesser, F., Scheffrahn, W., Pastorini, J., Vallet, D., Gaci, B., Martin, R. D., & Gautier-Hion, A. (2001). Is male-infant caretaking related to paternity and/or mating activities in wild Barbary macaques (Macaca sylvanus)? Comptes Rendus De L’académie Des Sciences III Vie, 324, 601–610. https://doi.org/10.1016/S0764-4469(01)01339-7
Mercier, S., Déaux, E. C., Waal, E. van de, Bono, A. E. J., & Zuberbühler, K. (2019). Correlates of social role and conflict severity in wild vervet monkey agonistic screams. PLOS ONE, 14(5), e0214640. https://doi.org/10.1371/journal.pone.0214640
Minge, C., Berghänel, A., Schülke, O., & Ostner, J. (2016). Patterns and Consequences of Male-Infant Relationships in Wild Assamese Macaques (Macaca assamensis). International Journal of Primatology, 37(3), 350–370. https://doi.org/10.1007/s10764-016-9904-2
Moscovice, L. R., Di Fiore, A., Crockford, C., Kitchen, D. M., Wittig, R., Seyfarth, R. M., & Cheney, D. L. (2010). Hedging their bets? Male and female chacma baboons form friendships based on likelihood of paternity. Animal Behaviour, 79(5), 1007–1015. https://doi.org/10.1016/j.anbehav.2010.01.013
Moscovice, L. R., Heesen, M., Di Fiore, A., Seyfarth, R., & Cheney, D. (2009). Paternity alone does not predict long-term investment in juveniles by male baboons. Behavioral Ecology and Sociobiology, 63(10), 1471–1482. https://doi.org/10.1007/s00265-009-0781-y
Murray, C. M., Stanton, M. A., Lonsdorf, E. V., Wroblewski, E. E., & Pusey, A. E. (2016). Chimpanzee fathers bias their behaviour towards their offspring. Royal Society Open Science, 3(11), 1–10. https://doi.org/10.1098/rsos.160441
Neumann, C., Agil, M., Widdig, A., & Engelhardt, A. (2013). Personality of Wild Male Crested Macaques (Macaca nigra). PLoS ONE, 8(8), e69383. https://doi.org/10.1371/journal.pone.0069383
Neumann, C., Duboscq, J., Dubuc, C., Ginting, A., Irwan, A. M., Agil, M., Widdig, A., & Engelhardt, A. (2011). Assessing dominance hierarchies: Validation and advantages of progressive evaluation with Elo-rating. Animal Behaviour, 82(4), 911–921. https://doi.org/10.1016/j.anbehav.2011.07.016
Neumann, C., Kulik, L., Agil, M., Engelhardt, A., & Widdig, A. (2022). Temporal dynamics and fitness consequences of coalition formation in male primates. Proceedings of the Royal Society b: Biological Sciences, 289(1976), 20212626. https://doi.org/10.1098/rspb.2021.2626
Newton-Fisher, N. E. (2017). Modeling Social Dominance: Elo-Ratings, Prior History, and the Intensity of Aggression. International Journal of Primatology, 38(3), 427–447. https://doi.org/10.1007/s10764-017-9952-2
Nguyen, N., van Horn, R., Alberts, S., & Altmann, J. (2009). “Friendships” between new mothers and adult males: Adaptive benefits and determinants in wild baboons (Papio cynocephalus). Behavioral Ecology and Sociobiology, 63(9), 1331–1344. https://doi.org/10.1007/s00265-009-0786-6
Nsubuga, A. M., Robbins, M. M., Roeder, A. D., Morin, P. A., Boesch, C., & Vigilant, L. (2004). Factors affecting the amount of genomic DNA extracted from ape faeces and the identification of an improved sample storage method. Molecular Ecology, 13, 2089–2094.
Ordóñez-Gómez, J. D., Dunn, J. C., Arroyo-Rodríguez, V., Méndez-Cárdenas, M. G., Márquez-Arias, A., & Santillán-Doherty, A. M. (2015). Role of Emitter and Severity of Aggression Influence the Agonistic Vocalizations of Geoffroy’s Spider Monkeys (Ateles geoffroyi). International Journal of Primatology, 36(2), 429–440. https://doi.org/10.1007/s10764-015-9833-5
Ostner, J., Vigilant, L., Bhagavatula, J., Franz, M., & Schülke, O. (2013). Stable heterosexual associations in a promiscuous primate. Animal Behaviour, 86(3), 623–631. https://doi.org/10.1016/j.anbehav.2013.07.004
Palombit, R. (2009). Friendships with males: A female counterstrategy to infanticide in chacma baboons of the Okavango Delta. In M. N. Muller & R. W. Wrangham (Eds.), Sexual coercion in primates and humans: An evolutionary perspective on male aggression against females (pp. 377–409). Harvard University Press.
Palombit, R. A., Cheney, D. L., Fischer, J., Johnson, S., Rendall, D., Seyfarth, R. M., & Silk, J. (2000). Male infanticide and defense of infants in chacma baboons. In C. P. van Schaik & C. H. Janson (Eds.), Infanticide by males and its implications (pp. 123–151). Cambridge University Press.
Paul, A., Kuester, J., & Arnemann, J. (1996). The sociobiology of male-infant interactions in Barbary macaques, Macaca sylvanus. Animal Behaviour, 51, 155–170. https://doi.org/10.1006/anbe.1996.0013
Petit, O., & Thierry, B. (1994). Aggressive and peaceful interventions in conflicts in Tonkean macaques. Animal Behaviour, 48(6), 1427–1436. https://doi.org/10.1006/anbe.1994.1378
Pusey, A. E., Williams, J., & Goodall, J. (1997). The influence of dominance rank on the reproductive success of female chimpanzees. Science, 277, 828–830. https://doi.org/10.1126/science.277.5327.828
R Core Team. (2017). R: A language and environment for statistical. R Foundation for Statistical Computing, Vienna, Austria.
Rosenbaum, S., Hirwa, J. P., Silk, J. B., Vigilant, L., & Stoinski, T. S. (2015). Male rank, not paternity, predicts male–immature relationships in mountain gorillas, Gorilla beringei beringei. Animal Behaviour, 104, 13–24. https://doi.org/10.1016/j.anbehav.2015.02.025
Schielzeth, H. (2010). Simple means to improve the interpretability of regression coefficients. Methods in Ecology and Evolution, 1(2), 103–113. https://doi.org/10.1111/j.2041-210X.2010.00012.x
Schielzeth, H., & Forstmeier, W. (2009). Conclusions beyond support: Overconfident estimates in mixed models. Behavioral Ecology, 20(2), 416–420. https://doi.org/10.1093/beheco/arn145
Silk, J. B. (2002). Using the’F’-word in primatology. Behaviour, 139(2/3), 421–446. https://doi.org/10.1163/156853902760102735
Smuts, B. B. (1985). Sex and friendship in baboons. Aldine.
Smuts, B. B., & Gubernick, D. J. (1992). Male-infant relationships in nonhuman primates: Paternal investment or mating effort? In B. S. Hewlett (Ed.), Father-child relations. Cultural and biosocial context. Aldine de Gruyter.
Storey, A. E., & Ziegler, T. E. (2016). Primate paternal care: Interactions between biology and social experience. Hormones and Behavior, 77, 260–271. https://doi.org/10.1016/j.yhbeh.2015.07.024
Taberlet, P., Griffin, S., Goossens, B., Questiau, S., Manceau, V., Escaravage, N., Waits, L. P., & Bouvet, J. (1996). Reliable genotyping of samples with very low DNA quantities using PCR. Nucleic Acids Research, 24(16), 3189–3194.
Taberlet, P., & Luikart, G. (1999). Non-invasive genetic sampling and individual identification. Biological Journal of the Linnean Society, 68(1–2), 41–55.
Thierry, Bynum, E. L., Baker, S., Kinnaird, M. F., Matsumura, S., Muroyama, Y., O’Brien, T. G., Petit, O., & Watanabe, K. (2000). The social repertoire of Sulawesi macaques. Primate Research, 16, 203–226.
Trivers, R. L. (1972). Parental investment and sexual selection. In B. Campbell (Ed.), Sexual selection and the descent of man (pp. 139–179). Aldine.
van Noordwijk, M. A., & van Schaik, C. P. (2000). Reproductive patterns in eutherian mammals: Adaptations against infanticide? In C. P. van Schaik & C. H. Janson (Eds.), Infanticide by Males and its Implications (pp. 322–360). Cambridge University Press. https://doi.org/10.1017/CBO9780511542312.016
van Schaik, C. P. (2000). Vulnerability to infanticide by males: Patterns among mammals. In C. P. van Schaik & C. H. Janson (Eds.), Infanticide by males and its implications (pp. 61–71). Cambridge University Press.
van Schaik, C. P., & Paul, A. (1996). Male care in primates: Does it ever reflect paternity? Evolutionary Anthropology, 5, 152–156.
Whitehead, H. (2008). Analyzing animal societies: Quantitative methods for vertebrate social analysis. University of Chicago Press.
Widdig, A. (2007). Paternal kin discrimination: The evidence and likely mechanisms. Biological Reviews, 82(2), 319–334. https://doi.org/10.1111/j.1469-185X.2007.00011.x
Acknowledgements
The authors thank the Indonesian government, particularly RISTEK and BKSDA, Manado for supporting this study. We are grateful to all field assistants, in particular Rebecca Gorman, Maria Panggur, the late Deidy Azhari, Nicole Thompson, supporting behavioural data collection and all members of the Macaca Nigra Project contributing to DNA sample collection. We also acknowledge Jan-Boje Pfeifer for his continuous logistic support of the project and Muhammad Agil for his support for permissions. Furthermore, we thank Kerstin Fuhrmann, Stefanie Bley and Maren Keller for conducting the genetic analysis and Linda Vigilant for providing laboratory access. The study was funded by the German Research Foundation within the Emmy-Noether programme (grant No. EN 719/1, 2 to AE, WI 1801/3-1 to AW) and the University of Leipzig (to AW), partly together with the German Federal Ministry for Economic Cooperation and Development (to AE). Moreover, we thank the Max-Planck Institute for Evolutionary Anthropology, the German Primate Center and the University of Leipzig for logistic support. Moreover, we thank Roger Mundry, Christof Neumann and Richard McElreath for statistical advice. Finally, Jérôme Micheletta, Brigitte M. Weiß, Joanna Setchell, and two reviewers for their helpful comments on an earlier version of the manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
AW and DK originally formulated the idea; DK conducted fieldwork, DPF, AE and AW generated paternity data, AE provided access to demographic data and coordinated fieldwork, LK and AW planned and performed the statistical analyses; DK, LK, AE, and AW wrote the manuscript, all authors approved the manuscript.
Corresponding author
Ethics declarations
Competing Interest
The authors declare no competing interests.
Inclusion and Diversity Statement
The author list includes contributors from the location where the research was conducted, who participated in study conception, study design, data collection, analysis, and interpretation of the findings.
Additional information
Handling Editor: Jerome Micheletta
Daphne Kerhoas, Lars Kulik equally contributed as first authors.
Antje Engelhardt, Anja Widdig equally contributed as senior authors.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kerhoas, D., Kulik, L., Perwitasari-Farajallah, D. et al. Do Wild, Male, Crested Macaques (Macaca nigra) Respond to the Screams of Infants Involved in Agonistic Interactions?. Int J Primatol 44, 626–648 (2023). https://doi.org/10.1007/s10764-023-00381-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-023-00381-8