Abstract
The role that single nutrients may play for food choices in nonhuman primates is not fully understood. White-faced sakis (Pithecia pithecia) are unusual among frugivorous primates as they do not serve as seed dispersers but rather exploit the seeds they consume, presumably for their high contents of lipids and proteins. Therefore, we assessed the occurrence of spontaneous food preferences in zoo-housed white-faced sakis and analyzed whether these preferences correlate with nutrient composition. Using a two-alternative choice test, we repeatedly presented three female and two male sakis with all possible binary combinations of 15 types of food that are part of their diet under human care, and found them to display the following rank order of preference: peanut > hazelnut > avocado > melon > egg > apple > mealworms > beetroot > carrot > cucumber > cabbage > tomato > sweet potato > broccoli > eggplant. This preference ranking significantly and positively correlated with the total energy content of the food items. However, we found the strongest positive correlation among the three macronutrients providing metabolic energy between the sakis’ food preferences and lipid content. This is remarkable as all other primate species tested so far using this method displayed the strongest correlation with carbohydrates instead. Together with our finding that the sakis significantly preferred foods high in mono-unsaturated fatty acids, the building blocks of lipids, these results support the notion that white-faced sakis exploit the lipids contained in seeds to meet their requirements of metabolic energy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primates feed on a wide variety of plant and animal matter to meet their nutritional requirements (Hohmann, 2009). However, numerous studies demonstrate that animals do not choose their food randomly, but instead are highly selective feeders in order to maximize their intake of critical nutrients (e.g., Chivers, 1998; Ganzhorn et al., 2017; Illius & Gordon, 1990; Raubenheimer & Rothman, 2013). It is commonly agreed that the food choices of primates and other mammals are primarily determined by the nutritional and/or toxic content of a specific plant or animal (Barton & Whiten, 1994; Windley et al., 2022) as well as by its relative temporal and spatial availability (Leighton, 1993; Trapanese et al., 2019). High concentrations of plant secondary compounds such as tannins, phenolics, or alkaloids which inhibit the digestion of proteins and polysaccharides or are even toxic, are sometimes avoided and, accordingly, correlate negatively with food choices in primates (Belovsky & Schmitz, 1994; Glander, 1982; Windley et al., 2022). High concentrations of nutritionally valuable compounds such as carbohydrates, proteins, and lipids which provide metabolic energy, or of certain critical minerals or vitamins, in contrast, should be expected to correlate positively with food choice (Lambert & Rothman, 2015; Simpson et al., 2004). Interestingly, only few studies in nonhuman primates so far reported such positive correlations between specific nutrients and food choice (e.g., Conklin-Brittain et al., 1998; Felton et al., 2009; Milton, 1998; Raubenheimer et al., 2015; Rothman et al., 2011). The reason for this may be that food items are usually composed of a mixture of both non-palatable or toxic compounds and attractive nutrients, and it is therefore difficult to disentangle whether a fruit, for example, is preferred due to its nutrient content or due to its low concentration of secondary compounds that plants may use as a defense against predation (Glander, 1982; Goyal et al., 2012).
Cultivated fruits and vegetables have been selectively bred to contain only negligible amounts of plant secondary compounds, making them safe and attractive for human consumption (Paliyath et al., 2008; Pott et al., 2019), and thus provide a useful option to clarify the roles of, e.g., sweet-tasting and thus attractive carbohydrates and bitter-tasting and thus non-attractive plant secondary compounds such as phenolics, alkaloids, and tannins for food selection. They also usually contain higher amounts of carbohydrates compared to the fruits consumed by primates in the wild (McLennan & Ganhorn, 2017; Bryson-Morrison et al., 2020). Furthermore, the nutrient composition of cultivated fruits and vegetables is well-established (Food Standards Agency, 2002) whereas that of plants consumed by primates in the wild is often unknown. Studies that adopted the approach to present cultivated fruits and vegetables to captive animals found that some primate species such as white-handed gibbons (Hylobates lar), ring-tailed lemurs (Lemur catta), and pigtail macaques (Macaca nemestrina) display significant positive correlations between their food preferences and carbohydrate content (Hansell et al., 2020; Jildmalm et al., 2008; Laska, 2001) and are thus selective feeders with regard to their preferred source of metabolic energy. Other primate species such as spider monkeys (Ateles geoffroyi) and squirrel monkeys (Saimiri sciureus), in contrast, displayed significant positive correlations with total energy content (Laska, 2001; Laska et al., 2000) and are thus opportunistic feeders with regard to their preferred source of metabolic energy. Nevertheless, among the three macronutrients that provide the bulk of metabolic energy — carbohydrates, proteins, and lipids — all primate species which were tested under controlled conditions and were presented with cultivated fruits and vegetables displayed the strongest positive correlations with carbohydrates.
White-faced sakis (Pithecia pithecia) are frugivorous platyrrhines whose diet comprises a variety of fruits, but also includes some leaves, insects, and flowers (Norconk & Setz, 2013). Whereas most other frugivorous primate species act as seed dispersers, sakis prey heavily upon the seeds of the fruits they consume and are thought to exploit the nutrients they contain (Norconk et al., 2013). Their dental morphology includes tusklike lower canines and scoop-like wedge lower incisors which are adapted for the extraction and mastication of seeds protected by tough outer membranes (Kinzey, 1992; Ledogar et al., 2013). Further, sakis have more robust mandibles compared to non-sclerocarpic frugivorous primates, allowing for the attachment of the massive muscles needed to provide the necessary pressure to crack open hard-shelled seeds (Anapol & Lee, 1994).
The average nutritional intake of lipids by white-faced sakis has been reported to be markedly higher than that of other frugivores, mostly due to the year-round consumption of young seeds from immature fruits that are especially high in lipid content (Norconk & Conklin-Brittain, 2004). Whereas seeds and arils consumed by white-faced sakis averaged lipid contents of > 20% of dry mass, with the arils of certain plant species even providing lipid contents > 60%, the fruit pulp as well as young and mature leaves consumed by Pithecia pithecia were uniformly low in lipid content (< 5% of dry mass). However, it is still unknown how lipids and other nutrients may affect food choice in white-faced sakis.
We assessed food preferences in a group of zoo-housed white-faced sakis for a variety of cultivated fruits and vegetables as well as for some foods of animal origin, and analyzed whether these preferences correlate with the contents of certain macro- and/or micronutrients of the food items used. We predicted that zoo-housed white-faced sakis (1.) should display marked preferences for certain types of food and that these preferences are based on their nutrient composition, and (2.) should display a preference for food items that are high in lipid content. Our first prediction is based on previous studies which reported that other species of captive nonhuman primates also display correlations between food preferences and nutrient composition, even though several driving factors of food selection such as seasonal fluctuations in the abundance, composition, and quality of available foods are much less pronounced under captive conditions compared to conditions in the wild. Our second prediction is based on previous studies that reported white-faced sakis in the wild to exploit the seeds of fruits they consume, presumably due to their high lipid content.
Methods
Animals
Five white-faced sakis (Pithecia pithecia), maintained at Furuviksparken (Furuvik, Sweden), participated in the study. They comprised two adult males (Kariakou and Engelbrekt, 16 and 10 years of age respectively) and three adult females (Lisha, Elin, and Anita, 14, 7, and 6 years of age respectively). All five animals were born in captivity. The sakis were housed in an indoor enclosure of 633 \({\mathrm{m}}^{3}\), with access to a 127 \({\mathrm{m}}^{3}\) outdoor enclosure. Their diet consisted of fruits and vegetables, primate extrudate high fiber pellets (from Granovit Zoofeed, Kaiseraugst, Switzerland) and a tamarin cake (from Mazuri Zoo Foods, Witham, Essex, Great Britain). Additionally, the animals had access to edible fresh leaves and branches that were placed in the enclosure occasionally. Fruits and vegetables were fed twice each day around 11:00 and 15:00, respectively, while water was always available ad libitum.
Procedures
We assessed food preferences using a two-alternative choice test. To this end, we presented the animals with pairs of food items and their choice behavior, i.e., which of the two food items was consumed, was recorded. We took care to present food items of approximately equal volume (~ 1 cm3) to minimize the risk that apparent differences in the size of the food items affected the animals’ food choices. We tested the sakis singly in order to avoid competition or distraction affecting an animal’s choice behavior.
We separated the animals for five test sessions each day, between 07:00 and 17:00. We took care that test sessions were not conducted immediately after the animals were presented with one of their two daily food rations to ensure that they had some appetite and motivation to participate. Accordingly, the minimum time period between the presentation of one of the two daily food rations and the start of a test session was 2 h. Each test session comprised a maximum of five pairwise presentations per animal and the position of the food items was pseudorandomized to counterbalance possible side preferences. To this end, we took care not to present a given food item more than three times in a row on the same side.
We presented all 105 possible binary combinations of 15 types of food for a total of ten times per animal and took care to never present a food item that had been part of the previous pair to prevent any bias. We cut all foods to (or presented them at) an equal size, approximating cubes with a side length of 2 cm to avoid choice behavior being affected by size differences.
In each session an animal voluntarily entered a testing cage (1 m × 2.4 m × 3 m) connected to the indoor enclosure through a sliding door. We tested all animals individually in order to prevent interference from, and distraction by, the other animals. The sakis then placed themselves on a wooden platform attached to a metallic mesh that separated the testing cage from the enclosure’s service area. We presented pairs of food items on a 30 × 22 cm cutting board which were covered by a box until its front edge came in contact with the mesh at the height of the wooden platform to ensure that the sakis were exposed to both food items simultaneously. This also stimulated the animals’ curiosity and maintained their motivation to participate in the tests. The mesh was wide enough to allow the sakis to fit their hand through and grab hold of a food item. As soon as an animal had decided for one of the two simultaneously presented food items by taking it, we removed the cutting board to prevent the animal from taking the other food item and their choice was recorded on paper.
The 15 different types of food employed were broccoli (Brassica oleracea var. botrytis), cucumber (Cucumis sativus), tomato (Lycopersicum esculentum), carrot (Daucus carota), eggplant (Solanum melongena), beetroot (Beta vulgaris), sweet potato (Ipomoea batatas), avocado (Persea americana), Napa cabbage (Brassica rapa, subsp. pekinensis), apple (Malus pumila), peanut (Arachis hypogaea), honey melon (Cucumis melo), hazelnut (Corylus avellana), mealworms (i.e., larvae of the mealworm beetle Tenebrio molitor), and hard-boiled egg (from chicken, Gallus gallus).
We presented both the peanuts and the hazelnuts as well as the hard-boiled eggs without their shells in order to minimize the risk that the expenditure of time needed for cracking and removing the shells might affect the animals’ food choices.
The rationale for choosing these types of food was that (a) all of them were part of the animals’ diet in captivity and thus familiar to the white-faced sakis and readily consumed when presented singly, (b) data for the contents of the macro- and micronutrients in these types of food are available, allowing us to assess possible correlations between food preferences and nutrient contents, expressed as proportion of edible matter (Food Standards Agency, 2002), and (c) they differ markedly in their content of macro- and/or micronutrients.
The contents of total energy, carbohydrates, lipids, protein, and water differed by up to a factor of 65, 210, 630, 64, and 21 respectively between the types of food used here. In an attempt to minimize the inevitable intraspecific variation in nutrient composition, we took care to always present food items of a given type with the same degree of ripeness and of the same variety or cultivar.
Data Analysis
We recorded a total of 5250 choices (105 binary combinations x ten presentations per animal x five animals), and we established food preference rankings using the following criteria (Hansell et al., 2020):
-
Criterion 1 (individual level). We built the sum total of choices for each of the 15 types of food across all binary combinations for each individual animal. The theoretical maximum score for any type of food with this criterion was 140 (14 combinations x ten presentations per animal x one animal). In cases when a saki failed to make a choice between two food items within 10 s, we assigned 0.5 points to each of the two items.
-
Criterion 2 (group level). This criterion adopts the same procedure of building the sum total of choices as for criterion 1, although, here, we collapsed the data for all five animals. Thus, the theoretical maximum score for any type of food with this criterion was 700 (14 combinations x ten presentations per animal x five animals).
We performed two-tailed binomial tests using the sum total of choices for each member of a given binary combination of food items to assess significant preferences both at the individual level and at the group level (p < 0.05). We evaluated correlations between the food preference rankings and the contents of nutrients by calculating Spearman rank-order correlation coefficients rs which we tested for significance by computing z-scores. We used the same test to assess whether the food preference rankings of the five sakis correlated with each other, and whether the food preference rankings of the males and the females correlated with each other. We performed all statistical tests with Bonferroni corrections for multiple testing.
Ethical Note
The experiments reported here comply with the American Society of Primatologists’ Principles for the Ethical Treatment of Primates, with the European Union Directive on the Protection of Animals Used for Scientific Purposes (EU Directive 2010/63/EU), and with current Swedish animal welfare laws.
Conflict of Interest
The authors declare that they have no conflict of interest.
Data Availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Results
Food Preferences
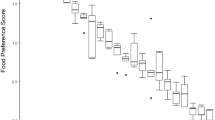
With 96 of the 105 binary combinations of food items that we presented to the animals, the white-faced sakis displayed a statistically significant preference for one of the options (two-tailed binomial test, p < 0.05) (Table I). Peanut was clearly the most attractive food and, accordingly, was significantly preferred over all 14 other food items (p < 0.05). The high attractiveness of peanut is further illustrated by the fact that 93.9% of all possible choices were in favour of this food item (Table II). Hazelnut and avocado were also significantly preferred over all other food items (with the obvious exception of peanut) and 89.9% and 88.4%, respectively, of all possible choices were in favour of these two food items. Eggplant and broccoli, in contrast, were the least attractive food items and were never significantly preferred over any of the other 13 food items. Accordingly, only 9.6% and 11.6%, respectively, of all possible choices were in favour of these two food items.
Rankings Derived from the Food Preferences
As a group, the white-faced sakis displayed the following rank order of preference: peanut > hazelnut > avocado > honey melon > egg > apple > mealworms > beetroot > carrot > cucumber > cabbage > tomato > sweet potato > broccoli > eggplant (Table II).
All five individual sakis displayed similar rankings of preference for the 15 food items. Accordingly, their food preference rankings all significantly correlated with each other (Spearman, N = 15, rs ≥ 0.91, p < 0.05, with all ten comparisons). The food preference rankings were also similar between the two males and the three females and, accordingly, correlated significantly with each other (Spearman, N = 15, rs = 0.98, p ˂ 0.05, with all four comparisons).
Food Preference Rankings and Nutritional Content
The food preference rankings displayed by the white-faced sakis correlated highly significantly with energy content of the food items (Table III). This was true both when the five sakis were considered separately and when they were considered as a group. We found highly significant negative correlations between the food preference ranking and the nutritional content of the food items with carotene and ascorbic acid (Table III). Additionally, we found significant positive correlations with mono-unsaturated fatty acids, riboflavin, vitamin E, biotin, copper, and iodine (Table III), and a significant negative correlation with water content (Table III).
Discussion
The results of the present study demonstrate that zoo-housed white-faced sakis display marked food preferences in a two-alternative choice test using cultivated fruits and vegetables as well as foods of animal origin. Further, the results show that these preferences significantly correlated positively with total energy content of the food items used.
Our finding that the sakis clearly preferred certain types of food over others, and that these preferences correlated with their content of certain nutrients, is in line with our first hypothesis. The optimal foraging theory predicts that natural selection should favor individuals that succeed in maximizing their intake of critical nutrients and this should be reflected in their food selection patterns and food preferences (Stephens & Krebs, 1986; Stephens et al., 2008). Therefore, it may not be surprising that the sakis in the present study were highly selective in their food choices, similar to other nonhuman primate species tested in previous studies adopting the same approach as the one employed here (Hansell et al., 2020; Jildmalm et al., 2008; Laska, 2001; Laska et al., 2000). Nevertheless, it is not self-evident that nutrient-based food preferences are not only found under natural, but also under captive conditions, considering that several driving factors of food selection such as seasonal fluctuations in the abundance, composition, and quality of available foods (Hemingway & Bynum, 2005) are much less pronounced, if present at all, in animals under human care. Rather, this finding emphasizes the usefulness of this approach for elucidating which nutrients may affect food choices in what way. Our approach may also form the basis for studies in which the food preferences displayed by captive animals may be compared to the food preferences displayed by their conspecifics in the wild. This would allow us to draw conclusions on whether, or to what degree, food preferences may mirror evolutionary adaptations to a species’ dietary specialization or are individually acquired.
Our finding that the food preferences displayed by the sakis significantly correlated positively with total energy content of the food items used appears to be in contrast with our second hypothesis. However, the food items with the highest amount of lipids per mass unit — hazelnut, peanut, avocado, mealworm, and egg — were all highly placed in the food preference ranking displayed by the sakis (1., 2., 3., 5., and 7., Table II). Accordingly, the strongest positive correlation among the three macronutrients providing metabolic energy, carbohydrates, proteins, and lipids respectively was between the sakis’ food preferences and lipid content (Table III). This is remarkable, as all captive primate species tested so far using the same approach as the one employed in the present study (squirrel monkeys and pigtail macaques: Laska, 2001; spider monkeys: Laska et al., 2000; white-handed gibbons: Jildmalm et al., 2008; ring-tailed lemurs: Hansell et al., 2020) as well as captive frugivorous rodents (pacas, Agouti paca: Laska et al., 2003) displayed the strongest positive correlation between their food preferences and carbohydrate content — as far as the three energy-bearing macronutrients are concerned. Thus, the present findings support the notion that sakis in the wild exploit the seeds they consume for their high lipid content (Norconk & Conklin-Brittain, 2004; Norconk et al., 2013). The presumed pivotal role of lipids for food choice in captive and, possibly, also in free-ranging sakis is further supported by our finding of a significant positive correlation between the sakis’ food preferences and the content of mono-unsaturated fatty acids (Table III) which are the building blocks of lipids.
Macronutrients
The exploitation of lipids is reasonable from an energetic point of view, as they provide 9 kcal/gram whereas both carbohydrates and proteins provide only 4 kcal/gram (Food Standards Agency, 2002). However, most primate diets contain very little fat, as leaves usually do not contain any measurable amounts of lipids and the pulp of most fruits consumed by primates contain only very low amounts (< 2% of dry mass) of lipids (Rothman et al., 2014).
The fact that most frugivorous primate species studied so far mainly rely on carbohydrates as their primary source of metabolic energy suggests that the effective exploitation of lipids may require anatomical and/or physiological evolutionary adaptations of the digestive system that are only favored under certain selective pressures and which may have acted upon the white-faced sakis (Lambert, 1998; Norconk et al., 2002). This notion is supported by the fact that white-faced sakis show both dental (Kinzey, 1992; Ledogar et al., 2013) and intestinal tract (Lambert, 1998; Norconk et al., 2002) adaptations which clearly distinguish this species from other, non-sclerocarpic frugivorous primates and which are consistent with the effective mastication and nutritional exploitation of hard-shelled seeds.
The lipid content of foods has also been reported to play a role in the food selection of some other primate species: folivorous black howler monkeys (Alouatta pigra), for example, were found to, at least seasonally, prefer foods high in lipid content (Righini et al., 2017), and frugivorous red-tailed monkeys (Cercopithecus ascanius) have been reported to prefer flowers of Symphonia globulifera which contain a markedly higher lipid content (14.8% of dry mass) compared to other flowers (1.4%) and other foods exploited in their habitat (Ross et al., 2022).
Although white-faced sakis include a high proportion of fruits into their diet (Norconk & Setz, 2013) and are therefore considered as frugivores, they are unusual among frugivorous primates not only by exploiting the seeds of the fruits they consume, but also by specializing on fruits that are unripe or at least not fully ripe (Norconk & Conklin-Brittain, 2004). Such fruits generally contain markedly lower amounts of soluble carbohydrates compared to fully ripe ones (Food Standards Agency, 2002), and it is thought that primates specializing on unripe fruit do so to avoid competition with sympatric frugivores (Garber, 1987; Stevenson et al., 2000). Our finding that the sakis of the present study did not show a significant correlation between their food preferences and the content of carbohydrates (Table III), which furthermore, was markedly weaker compared to the correlation with lipids and mono-unsaturated fatty acids, suggests that they may rely more on the lipids contained in the seeds of the fruits they consume than on the carbohydrates contained in the pulp of these fruits to meet their requirements of metabolic energy.
The diet of frugivorous primates is usually considered to be low in protein content, at least compared to the diet of insectivores and folivores (Ganzhorn et al., 2017; Hohmann, 2009). However, the seeds that white-faced sakis consume contain clearly higher amounts of protein than the pulp of the seed-bearing fruits that non-sclerocarpic frugivores feed on (Norconk & Conklin-Brittain, 2004). This is in line with our finding that the sakis displayed a stronger, though statistically not significant, positive correlation between their food preferences and protein content (Table III) compared to all other primate species tested so far which do not exploit seeds (squirrel monkeys and pigtail macaques: Laska, 2001; spider monkeys: Laska et al., 2000; white-handed gibbons: Jildmalm et al., 2008; ring-tailed lemurs: Hansell et al., 2020). Frugivorous primates that do not exploit the nutrients contained in seeds are thought to meet their protein requirements by consuming animal matter such as arthropods, either by actively foraging for them (Risch Ferreira et al., 2021) or by selecting fruits that are infested with insect larvae (dos Santos-Barret et al., 2022).
White-faced sakis have been reported to regularly drink from open water sources such as tree cisterns (Cunningham & Janson, 2013). Without having to rely on water-rich food items to meet their water requirements, they can therefore prioritize food items with higher energy values which are typically low in water content (Food Standards Agency, 2002). This, in turn, fits to our finding of a significant negative correlation between the sakis’ food preferences and water content of the food items tested (Table III). Nevertheless, we cannot exclude the possibility that this finding may be due to the ad-libitum access to water in our zoo-housed sakis. Previous studies showed that spider monkeys (Laska et al., 2000) and squirrel monkeys (Laska, 2001), too, displayed such a significant negative correlation with water content, whereas pigtail macaques, white-handed gibbons, and ring-tailed lemurs (Hansell et al., 2020; Jildmalm et al., 2008; Laska, 2001) did not. Future studies should therefore further assess whether food preferences in primates reflect a possible trade-off between the needs to meet their water and their energy requirements.
Micronutrients
It is well-established that animals are capable of selectively preferring foods that supply certain micronutrients such as minerals or vitamins to counterbalance the lack of a given micronutrient in their diet (Simpson et al., 2004). Minerals such as sodium, copper, and iron are usually not abundant in the leaves and fruits consumed by primates, and may thus be limiting factors in primate diets (Rothman et al., 2014). In order to prevent possible deficiencies in certain minerals, primates in the wild have repeatedly been reported to engage in geophagy and in using mineral licks (Ferrari et al., 2008; Krishnamani & Mahaney, 2000). Both behaviors have also been reported in free-ranging sakis (Setz et al., 1999). We found that the white-faced sakis of the present study displayed significant positive correlations between their food preferences and the contents of copper and iodine, respectively (Table III). At this point, it is difficult to decide whether this finding may be indicative of a lack of these minerals in the diet provided to our zoo-housed animals, or whether the contents of these two minerals simply correlated with the contents of another nutrient and that the corresponding preference for the mineral may thus be a by-product of a preference for this other nutrient. Nevertheless, two other platyrrhine primate species, spider monkeys (Laska et al., 2000) and squirrel monkeys (Laska, 2001), also displayed significant positive correlations between their food preferences and copper content of the food items tested, whereas three catarrhine primate species, pigtail macaques (Laska, 2001), white-handed gibbons (Jildmalm et al., 2008) and ring-tailed lemurs (Hansell et al., 2020) did not. Considering that copper deficiency in nonhuman primates has been associated with osteoporosis, cardiovascular disease, and poor immune response (Lopez de Romana et al., 2011) future studies on the diet of platyrrhine primates under human care should therefore consider a sufficient supply of this trace mineral.
We also found that the sakis displayed significant positive correlations between their food preferences and the contents of the vitamins A and C, and significant negative correlations with the vitamins B2, E, and H (Table III). Here, too, it is difficult to decide whether these findings may indicate a lack (or oversupply) of these vitamins in the diet of our zoo-housed animals or whether they are just a by-product of a preference for another nutrient. Interestingly, none of the other primate species tested previously with the same approach as used in the present study showed any significant correlations between their food preferences and the contents of a vitamin (spider monkeys: Laska et al., 2000; squirrel monkeys and pigtail macaques: Laska, 2001; white-handed gibbons: Jildmalm et al., 2008; ring-tailed lemurs: Hansell et al., 2020). As captive nonhuman primates, including white-faced sakis, have been reported to be susceptible to both vitamin deficiencies and toxicities (Crissey & Pribyl, 2000; Minich et al., 2022) future studies should therefore carefully monitor the amounts of vitamins in the diet of captive primates.
Limitations of Our Study
Our sample size of five animals was rather small. Accordingly, our findings do not allow for generalizations to the species level. This is particularly true as we employed zoo-housed animals and not animals in the wild. Similarly, our study included repeated presentation of the same pairs of food items to our small study population. This entails the problem of pseudo-replication. However, the scientific question we addressed — whether zoo-housed white-faced sakis display food preferences and whether these correlate with the contents of certain nutrients — inevitably requires repeated testing in order to assess whether the preference for a given food item displayed by a an animal is stable across trials. Accordingly, even if we had access to a larger study population we would have needed to perform repeated presentation of the same pairs of food items to control for external factors which may affect the animals’ food choices.
In summary, we found that zoo-housed white-faced sakis displayed marked food preferences in a two-alternative choice test using cultivated fruits and vegetables as well as foods of animal origin. These preferences significantly correlated positively with total energy content of the food items used. However, the strongest positive correlation among the three macronutrients providing metabolic energy was between the sakis’ food preferences and lipid content. This is remarkable as all other primate species tested so far displayed the strongest correlation with carbohydrates instead. Together with our finding that the sakis significantly preferred foods high in mono-unsaturated fatty acids, the building blocks of lipids, these results support the notion that white-faced sakis exploit the energy provided by the lipids contained in seeds.
References
Anapol, F., & Lee, S. (1994). Morphological adaptation to diet in platyrrhine primates. American Journal of Physical Anthropology, 94, 239–261.
Barton, R. A., & Whiten, A. (1994). Reducing complex diets to simple rules: Food selection by olive baboons. Behavioral Ecology and Sociobiology, 35, 283–293.
Belovsky, G. E., & Schmitz, O. J. (1994). Plant defenses and optimal foraging by mammalian herbivores. Journal of Mammalogy, 75, 816–832.
Bryson-Morrison, N., Beer, A., Soumah, A. G., Matsuzawa, T., & Humle, T. (2020). The macronutrient composition of wild and cultivated foods of West African chimpanzees (Pan troglodytes verus) inhabiting an anthropogenic landscape. American Journal of Primatology, 82, e23102.
Chivers, D. J. (1998). Measuring food intake in wild animals: Primates. Proceedings of the Nutrition Society, 57, 321–332.
Conklin-Brittain, M. L., Wrangham, R., & Hunt, K. D. (1998). Dietary response of chimpanzees and cercopithecines to seasonal variation in fruit abundance: II. Macronutrients. International Journal of Primatology, 19, 971–998.
Crissey, S., & Pribyl, L. (2000). A review of nutritional deficiencies and toxicities in captive New World primates. International Zoo Yearbook, 37, 355–360.
Cunningham, E. P., & Janson, C. H. (2013). Effect of fruit scarcity on use of spatial memory in a seed predator, white-faced saki (Pithecia pithecia). International Journal of Primatology, 34, 808–822.
dos Santos-Barret, T. C., Cavalcante, T., Boyle, S. A., Matte, A. L., Bezerra, B. M., de Oliveira, T. G., & Barnett, A. A. (2022). Pulp fiction: Why some populations of ripe-fruit specialists Ateles chamek and A. marginatus prefer insect-infested foods. International Journal of Primatology, 43, 384–408.
Felton, A. M., Felton, A., Raubenheimer, D., Simpson, S. J., Foley, W. J., Wood, J. T., Wallis, I. R., & Lindenmayer, D. B. (2009). Protein content of diets dictates the daily energy intake of a free-ranging primate. Behavioral Ecology, 20, 685–690.
Ferrari, S. F., Veiga, L. M., & Urbani, B. (2008). Geophagy in New World monkeys (Platyrrhini): Ecological and geographic patterns. Folia Primatologica, 79, 402–415.
Food Standards Agency. (2002). McCance and Widdowson’s the composition of foods (6th ed.). Royal Society of Chemistry.
Ganzhorn, J. U., Arrigo-Nelson, S. J., Carrai, V., Chalise, M. K., Donati, G., Droescher, I., Eppley, T. M., Irwin, M. T., Koch, F., Koenig, A., Kowalewski, M. M., Mowry, C., Patel, E. R., Pichon, C., Ralison, J., Reissdorf, C., Simmen, B., Stalenberg, E., Starrs, D., … Foley, W. J. (2017). The importance of protein in leaf selection of folivorous primates. American Journal of Primatology, 78, e22550.
Garber, P. A. (1987). Foraging strategies among living primates. Annual Review of Anthropology, 16, 339–364.
Glander, K. E. (1982). The impact of plant secondary compounds on primate feeding behavior. American Journal of Physical Anthropology, 25, 1–18.
Goyal, S., Lambert, C., Cluzet, S., Mérillon, J. M., & Ramawat, K. G. (2012). Secondary metabolites and plant defence. In J. M. Mérillon & K. G. Ramawat (Eds.), Plant defence: Biological control (pp. 109–138). Springer.
Hansell, M., Åsberg, A., & Laska, M. (2020). Food preferences and nutrient composition in zoo-housed ring-tailed lemurs, Lemur catta. Physiology & Behavior, 226, 113125.
Hemingway, C. A., & Bynum, N. (2005). The influence of seasonality on primate diet and foraging. In D. K. Brockman & C. P. van Schaik (Eds.), Seasonality in primates (pp. 57–104). Cambridge University Press.
Hohmann, G. (2009). The diet of non-human primates: Frugivory, food processing, and food sharing. In J. J. Hublin & M. P. Richards (Eds.), The evolution of hominin diets (pp. 1–14). Springer.
Illius, A. W., & Gordon, I. J. (1990). Constraints on diet selection and foraging behavior in mammalian herbivores. In R. N. Hughes (Ed.), Behavioural mechanisms of food selection (pp. 369–392). Springer.
Jildmalm, R., Amundin, M., & Laska, M. (2008). Food preferences and nutrient composition in captive white-handed gibbons, Hylobates lar. International Journal of Primatology, 29, 1535–1547.
Kinzey, W. G. (1992). Dietary and dental adaptations in the Pitheciinae. American Journal of Physical Anthropology, 88, 499–514.
Krishnamani, R., & Mahaney, W. C. (2000). Geophagy among primates: Adaptive significance and ecological consequences. Animal Behaviour, 59, 899–915.
Lambert, J. E. (1998). Primate digestion: Interactions among anatomy, physiology, and feeding ecology. Evolutionary Anthropology, 7, 8–20.
Lambert, J. E., & Rothman, J. E. (2015). Fallback foods, optimal diets, and nutritional targets: Primate responses to varying food availability and quality. Annual Review of Anthropology, 44, 493–512.
Laska, M. (2001). A comparison of food preferences and nutrient composition in captive squirrel monkeys, Saimiri sciureus, and pigtail macaques, Macaca nemestrina. Physiology & Behavior, 73, 111–120.
Laska, M., Hernandez Salazar, L. T., & Rodriguez Luna, E. (2000). Food preferences and nutrient composition in captive spider monkeys, Ateles geoffroyi. International Journal of Primatology, 21, 671–683.
Laska, M., Luna Baltazar, J. M., & Rodriguez Luna, E. (2003). Food preferences and nutrient composition in captive pacas, Agouti paca (Rodentia, Dasyproctidae). Mammalian Biology, 68, 31–41.
Ledogar, J. A., Winchester, J. M., St. Clair, E. M., & Boyer, D. M. (2013). Diet and dental topography in Pitheciine seed predators. American Journal of Physical Anthropology, 150, 107–121
Leighton, M. (1993). Modeling dietary selectivity by Bornean orangutans: Evidence for integration of multiple criteria in fruit selection. International Journal of Primatology, 14, 257–311.
Lopez de Romana, D., Olivares, M., Uauy, R., & Araya, M. (2011). Risks and benefits of copper in light of new insights of copper homeostasis. Journal of Trace Elements in Medicine and Biology, 25, 3–13.
McLennan, M. R., & Ganhorn, J. U. (2017). Nutritional characteristics of wild and cultivated foods for chimpanzees (Pan troglodytes) in agricultural landscapes. International Journal of Primatology, 38, 122–150.
Milton, K. (1998). Physiological ecology of howlers (Alouatta): Energetic and digestive considerations and comparison with the Colobinae. International Journal of Primatology, 19, 513–548.
Minich, D. J., Henry, B. A., & Levens, G. P. (2022). Metabolic bone disease in a white-faced saki (Pithecia pithecia). Veterinary Record Case Reports, 2022, e393.
Norconk, M. A., & Conklin-Brittain, N. L. (2004). Variation on frugivory: The diet of Venezuelan white-faced sakis. International Journal of Primatology, 25, 1–26.
Norconk, M. A., Grafton, B. W., & McGraw, W. S. (2013). Morphological and ecological adaptations to seed predation — a primate-wide perspective. In L. M. Veiga, A. A. Barnett, S. F. Ferrari, & M. A. Norconk (Eds.), Evolutionary biology and conservation of titis, sakis and uacaris (pp. 55–71). Cambridge University Press.
Norconk, M. A., Oftedal, O. T., Power, M. L., Jakubasz, M., & Savage, A. (2002). Digesta passage and fiber digestibility in captive white-faced sakis (Pithecia pithecia). American Journal of Primatology, 58, 23–34.
Norconk, M. A., & Setz, E. Z. (2013). Ecology and behavior of saki monkeys (genus Pithecia). In L. M. Veiga, A. A. Barnett, S. F. Ferrari, & M. A. Norconk (Eds.), Evolutionary biology and conservation of titis, sakis and uacaris (pp. 262–271). Cambridge University Press.
Paliyath, G., Murr, D. P., Handa, A. K., & Lurie, S. (2008). Postharvest biology and technology of fruits, vegetables, and flowers. Wiley.
Pott, D. M., Osorio, S., & Vallarino, J. G. (2019). From central to specialized metabolism: An overview of some secondary compounds derived from the primary metabolism for their role in conferring nutritional and organoleptic characteristics to fruit. Frontiers in Plant Science, 10, 835.
Raubenheimer, D., Machovsky-Capuska, G. E., Chapman, C. A., & Rothman, J. M. (2015). Geometry of nutrition in field studies: An illustration using wild primates. Oecologia, 177, 223–234.
Raubenheimer, D., & Rothman, J. M. (2013). Nutritional ecology of entomophagy in humans and other primates. Annual Review of Entomology, 58, 141–160.
Righini, N., Garber, P. A., & Rothman, J. M. (2017). The effects of plant nutritional chemistry on food selection of Mexican black howler monkeys (Alouatta pigra): The role of lipids. American Journal of Primatology, 79, e22524.
Risch Ferreira, N. I., Verhaagh, M., & Heymann, E. W. (2021). Myrmecovory in neotropical primates. Primates, 62, 871–877.
Ross, A. C., Bryer, M. A. H., Chapman, C. A., Rothman, J. M., Nevo, O., & Valenta, K. (2022). Why eat flowers? Symphonia globulifera flowers provide a fatty resource for red-tailed monkeys. Folia Primatologica, 93, 41–52.
Rothman, J. M., Raubenheimer, D., Bryer, M. A. H., Takahashi, M., & Gilbert, C. C. (2014). Nutritional contributions of insects to primate diets: Implications for primate evolution. Journal of Human Evolution, 71, 59–69.
Rothman, J. M., Raubenheimer, D., & Chapman, C. A. (2011). Nutritional geometry: Gorillas prioritize non-protein energy while consuming surplus protein. Biology Letters, 7, 847–849.
Setz, E. Z. F., Enzweiler, J., Solferini, V. N., Amendola, M. P., & Berton, R. S. (1999). Geophagy in the golden-faced saki monkey (Pithecia pithecia chrysocephala) in the Central Amazon. Journal of Zoology, 247, 91–103.
Simpson, S. J., Sibly, R. M., Lee, K. P., Behmer, S. T., & Raubenheimer, D. (2004). Optimal foraging when regulating intake of multiple nutrients. Animal Behaviour, 68, 1299–1311.
Stephens, D. W., & Krebs, J. R. (1986). Foraging theory. Princeton University Press.
Stephens, D. W., Brown, J. S., & Ydenberg, R. C. (2008). Foraging behavior and ecology. University of Chicago Press.
Stevenson, P. R., Quinones, M. J., & Ahumada, J. A. (2000). Influence of fruit availability on ecological overlap among four neotropical primates at Tinigua National Park, Colombia. Biotropica, 32, 533–544.
Trapanese, C., Meunier, H., & Masi, S. (2019). What, where and when: Spatial foraging decisions in primates. Biological Reviews, 94, 483–502.
Windley, H. R., Starrs, D., Stalenberg, E., Rothman, J. M., Ganzhorn, J. U., & Foley, W. J. (2022). Plant secondary metabolites and primate food choices: A meta-analysis and future directions. American Journal of Primatology, 2022, e23397.
Acknowledgements
The authors gratefully acknowledge the help of the primate caretakers at Furuviksparken. We also acknowledge the editor and the reviewers for their comments on the manuscript.
Funding
Open access funding provided by Linköping University.
Author information
Authors and Affiliations
Contributions
VAM and ML conceived and designed the experiments. VAM performed the experiments. VAM, ML, NM analyzed the data. VAM, ML, NM wrote the manuscript.
Corresponding author
Additional information
Handling Editor: Joanna (Jo) M. Setchell
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Martins, V.A., Magnusson, N. & Laska, M. Go for Lipids! Food Preferences and Nutrient Composition in Zoo-Housed White-Faced Sakis, Pithecia pithecia. Int J Primatol 44, 341–356 (2023). https://doi.org/10.1007/s10764-022-00344-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-022-00344-5