Abstract
Ecology is fundamental in the development, transmission, and perpetuity of primate technology. Previous studies on tool site selection have addressed the relevance of targeted resources and raw materials for tools, but few have considered the broader foraging landscape. In this landscape-scale study of the ecological contexts of wild chimpanzee (Pan troglodytes verus) tool use, we investigated the conditions required for nut-cracking to occur and persist in discrete locations at the long-term field site of Bossou, Guinea. We examined this at three levels: selection, frequency of use, and inactivity. We collected data on plant foods, nut trees, and raw materials using transect and quadrat methods, and conducted forest-wide surveys to map the location of nests and watercourses. We analysed data at the quadrat level (n = 82) using generalised linear models and descriptive statistics. We found that, further to the presence of a nut tree and availability of raw materials, abundance of food-providing trees as well as proximity to nest sites were significant predictors of nut-cracking occurrence. This suggests that the spatial distribution of nut-cracking sites is mediated by the broader behavioural landscape and is influenced by non-extractive foraging of perennial resources and non-foraging activities. Additionally, the number of functional tools was greater at sites with higher nut-cracking frequency, and was negatively correlated with site inactivity. Our research indicates that the technological landscape of Bossou chimpanzees shares affinities with the ‘favoured places’ model of hominin site formation, providing a comparative framework for reconstructing landscape-scale patterns of ancient human behaviour. A French translation of this abstract is provided in theelectronic supplementary information: EMS 2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The local environment plays an important role in shaping non-human primate behaviour from foraging strategies, to ranging patterns, and sociality (Robbins & Hohmann, 2006; Strier, 2011). Tool-use, particularly for extractive foraging, is no exception. Recent studies have highlighted that ecology is key in determining whether tool-use emerges in a population, how it manifests itself, and how it is maintained once it is established (S. Carvalho et al., 2007, 2011; Grund et al., 2019; Koops et al., 2013, 2014).
Stone tool-use is often recurrent in spatially discrete locations, frequently involves the reuse of tools, and leaves a recognisable archaeological footprint that can be traced back thousands of years (S. Carvalho et al., 2009; Falótico et al., 2019; Mercader et al., 2007). However, little is known about the ecological factors influencing selection and repeated use of specific locations for these activities and how they fit within the broader foraging landscape. Lithic-based foraging technology has been observed in several wild, non-human primate species including chimpanzees (Pan troglodytes verus and P. t. ellioti, formerly P.t. vellerosus; Whiten et al., 1999; Abwe & Morgan, 2008), bearded capuchin monkeys (Sapajus libidinosus; Ottoni & Izar, 2008), Burmese long-tailed macaques (Macaca fascicularis; Gumert et al., 2009), and, most recently, in white-faced capuchins (Cebus capuchinus; Barrett et al., 2018).
Chimpanzees are of particular interest because they are one of our closest living relatives (Langergraber et al., 2012), and they present one of the largest, most diverse and ecologically adaptable technological repertoire among non-human species, reflecting a level of cognitive flexibility akin to the earliest hominin toolmakers (S. Carvalho et al., 2013; Pascual-Garrido & Almeida-Warren, 2021; Rolian & Carvalho, 2017). Chimpanzee nut-cracking assemblages are very similar to the low-density assemblages characteristic of the early hominin record (S. Carvalho et al., 2008; S. Carvalho & McGrew, 2012). Thus, understanding how patterns of nut-cracking behaviour accumulate across the landscape can provide valuable insights into the formation and spatial distribution of early hominin assemblages, and allow the modelling of ancient landscape use and resource exploitation.
Research on chimpanzee nut-cracking has established that the spatial availability of nut trees and raw materials for tools influences site location and reuse, as well as frequency and distance of tool transport (Boesch & Boesch, 1984; S. Carvalho et al., 2007, 2011; Luncz et al., 2016). Nevertheless, nut-cracking assemblages are yet to be explored within the context of the broader ecological and foraging landscape. This requires the study of nut-cracking sites not only in relation to direct ecological correlates such as access to raw materials and nuts, but also in relation to ecological requirements of other daily activities critical to survival such as food, water, and shelter.
Chimpanzees living in forested environments spend approximately 50% of their waking hours foraging and travelling between feeding locations (Pruetz & Bertolani, 2009). Studies have shown that chimpanzee ranging patterns are dynamic and are affected by the spatial distribution and seasonality of food (Trapanese et al., 2019; Wrangham, 1977), which may also influence where non-foraging activities, such as nesting, take place (Basabose & Yamagiwa, 2002; Hernandez-Aguilar, 2009; Janmaat et al., 2014). Their diet mainly consists of fruit, followed by leaves and terrestrial herbs (Goodall, 1963; Morgan & Sanz, 2006), but high-energy foods such as insects, nuts, and honey acquired through tool-assisted foraging are also important staples or nutritional supplements for many chimpanzee populations (Sanz & Morgan, 2013). Yet, little is known about how extractive foraging interacts with other feeding activities and the broader behavioural landscape.
Water is essential to life (Popkin et al., 2010). For savannah-dwelling chimpanzees living in extremely arid conditions, water is a critical resource that constrains movement patterns and landscape use (Lindshield et al., 2021; Pruetz & Herzog, 2017; Wessling, Deschner, et al., 2018a; Wessling, Kühl, et al., 2018b). At face value, water provides hydration and a medium in which primates, and other living organisms, can stay cool. However, when examined in the broader foraging and behavioural context, other equally important qualities emerge. Most notably, water sustains plant species. These not only give shade but also provide a range of foods, some of which may not be encountered elsewhere. For some chimpanzee populations, water is also a source of aquatic resources such as algae (Boesch, Bombjaková, et al., 2017; Humle & Matsuzawa, 2009) and fresh-water crabs (Koops et al., 2019). Finally, free-flowing water can transport stone cobbles, and therefore serve as a source of raw materials for stone tools. This relationship has been proposed to explain the correlation between chimpanzee nut-cracking sites and hydrological features in Diécké, Guinea (Carvalho, 2011; Carvalho et al., 2007), although this remains to be empirically tested.
Chimpanzees habitually make a sleeping nest at the end of every day, and sometimes make day nests for resting (Koops et al., 2012). They have been an important focus of research since Sept (1992) recognised that they form clusters of debris akin to early hominin assemblages, and saw their potential for understanding patterns of early hominin landscape use and the origins of human shelter (McGrew, 2021). Nesting locations have subsequently been linked with a range of ecological parameters such as tree species and tree architecture, as well as surrounding topography and vegetation types (Badji et al., 2018; J. S. Carvalho et al., 2015; Hernandez-Aguilar, 2009; Hernandez-Aguilar et al., 2013; Koops et al., 2012; Ndiaye et al., 2018; Stewart et al., 2011). Other studies have found additional links between nest sites and proximity to areas of high fruit availability (Basabose & Yamagiwa, 2002; Furuichi & Hashimoto, 2004; Goodall, 1962; Janmaat et al., 2014), and that these correlations are not universal (Koops et al., 2012). While the exact conditions determining nest site suitability appear to be population-specific, these findings demonstrate that nesting activities shape chimpanzee landscape use, and, in turn, are shaped by resource distribution and the local environment. Nevertheless, little is known about how nesting relates to other activities such as spatially discreet forms of tool use (e.g., nut-cracking and termite-fishing).
Understanding how technological landscapes are shaped by the broader behavioural and ecological contexts can be dissected into three key points of inquiry: 1) tool site selection — where tool use occurs, 2) tool site use — how often tool use takes place at these locations, and 3) tool site inactivity — what conditions lead to the abandonment of a tool site. Studying the spatial ecology of technical activities is crucial to understanding how tool sites emerge in spatially discrete locations. There is a growing body of research on differential habitat use for daily activities such as foraging, travelling, socializing, and sleeping, particularly in relation to human-modified landscapes and implications for conservation (Bryson-Morrison et al., 2017; Potts et al., 2016). With regard to technological activities, we are also beginning to learn about raw material selection for tool use and manufacture (Carvalho et al., 2008; Pascual-Garrido & Almeida-Warren, 2021), yet how such ecological variables may affect the selection of locations for tool use remains largely unexplored.
How often a site is used can serve as a proxy for inferring preference in relation to other sites. From an archaeological perspective, generally the more a location is used for debris-generating activities, the larger and more conspicuous an archaeological signature becomes. This has important implications for understanding the spatial clustering of activities and material evidence over time, and can offer many insights into the formation and perpetuity of early hominin archaeological assemblages (Carvalho & Almeida-Warren, 2019; McGrew, 2010). Ethnoarchaeological studies of chimpanzee nests have found that sleeping sites are frequently revisited, and the nests themselves may be reused (Hernandez-Aguilar, 2009; Sept, 1992; Stewart et al., 2011). However, comparable literature on the use of tool sites is scarce, except for the reuse of stone tools (Carvalho et al., 2009), and sources of perishable raw materials (Pascual-Garrido, 2018; Pascual-Garrido & Almeida-Warren, 2021).
Chimpanzees live in dynamic landscapes shaped by environmental change. Whether natural or anthropogenic, these shifts often result in changes in resource distributions that may in turn lead to the development of new behavioural adaptations and ranging patterns (e.g., Kalan et al., 2020). New opportunities may arise (e.g., foraging food from crops: Hockings et al., 2015, Hockings et al., 2012; nesting in novel plant species: McCarthy et al., 2017), while formerly habitual activities may shift to new locations or become obsolete (Gruber et al., 2012; Kühl et al., 2019). Environmental changes have been identified as important drivers of our own evolutionary history (Bobe et al., 2002; Bobe & Carvalho, 2019; Joordens et al., 2019; R. Potts, 1998; Potts et al., 2020; Reed, 1997), but few studies have addressed empirically how these changes may have affected patterns of landscape use and the distribution of early hominin tool sites (e.g., Rogers et al., 1994). Thus, investigating the conditions that influence the cessation of activity at chimpanzee tool sites, can provide important clues as to the factors that lead to their temporary or long-term abandonment.
In this study, we explore the relationship between tool use and the broader behavioural and ecological landscapes by investigating the ecological drivers of chimpanzee nut-cracking at the long-term field site of Bossou (Guinea). We examine, at a landscape-scale, the conditions required for nut-cracking to occur and persist over time in spatially discrete locations, termed tool sites. To achieve this, we divided our investigation into three steps: tool site selection, tool site use, and tool site inactivity. For each of these steps, we assess the effect of ecological parameters that have been found to correlate with nut-cracking activities (nut availability; abundance of raw materials; distance to water) as well as variables that encapsulate two key aspects of chimpanzee activity patterns: non-extractive foraging (abundance of food resources: wild food trees; terrestrial herbaceous vegetation — THV) and sleeping (distance to nesting site). We employed archaeological and ecological methods for data collection and performed our analyses within an exploratory framework.
Methods
Study site and subjects
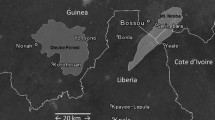
Bossou (7° 39′ N, 8° 30′ W) is located in the southeasternmost corner of the Republic of Guinea (West Africa), 6 km from the foothills of Mount Nimba Strict Nature Reserve (Fig. 1) (Humle, 2011a; Yamakoshi & Sugiyama, 1995). The chimpanzee community has been studied continuously in both natural (since 1976) and experimental (outdoor laboratory since 1988) settings (Matsuzawa, 2011 ; Sugiyama & Koman, 1979). Between 1976 and 2003 the community was composed of 18 to 23 individuals (Sugiyama, 2004), but has since declined largely due to a catastrophic flu-like epidemic from which it never recovered (Humle, 2011b; Sugiyama & Fujita, 2011). At the time of this study, the population consisted of seven individuals. The Bossou chimpanzees habitually crack and consume oil-palm nuts (Elaeis guineensis) and are currently one of only three communities known to use portable stones as both hammers and anvils (Ohashi, 2015). Bossou has two seasons — a short dry season lasting from November to February, and a long rainy season extending from March to October (Humle, 2011a; Yamakoshi, 1998). Nut-cracking occurs year-round, but is most prevalent during peak wet season (June–August) and at the start of the dry season (November–December) when fruit is less abundant (Yamakoshi, 1998).
Map of the Bossou Forest and surrounding area. Mount Nimba Strict Nature Reserve shapefile adapted from the WDPA database (UNEP-WCMC and IUCN, 2019).
The Bossou forest has an estimated area of 16 km2 and is intersected by roads (Fig. 2; Hockings et al., 2006). Within this, the chimpanzees range in a core area of approximately 7 km2 (Hockings et al., 2006). The habitat is comprised of a composite of primary, secondary, and riverine forests, savannah, and cultivated fields (Hockings et al., 2012). The northern slope of Mont Gban, in the eastern part of the forest, is considered a sacred area — forêt sacrée — by the local Manon culture, and access is forbidden to outsiders. Out of respect to this tradition, we did not conduct any research in this area.
Data collection
We collected data over two field trips: 14 December 2017 to 01 May 2018 and 28 October 2018 to 13 December 2018, encompassing 160 days of fieldwork. We employed a mixed-method approach that combined direct behavioural observation through group follows of the chimpanzee community, with archaeological documentation of nut-cracking sites (indirect behavioural observations; as per S. Carvalho & Almeida-Warren, 2019), and ecological research using the transect and quadrat method (as per S. Carvalho et al., 2011). Initially, we targeted nut-cracking sites that had previously been documented by SC in 2006 and 2008–2009, and expanded this sample to include any new nut-cracking sites that were discovered during the ecological surveys and group follows. For each nut-cracking site, we established 1-km transects intersecting the site datum at 500 m. However, if a nut-cracking sites was located within 100 m of a pre-established transect, we either assigned it to that transect or made it the mid-point of a new perpendicular transect in cases where those areas had not yet been recorded, to ensure even forest coverage. Our transects (n = 18) were oriented N–S or E–W, except for two that were oriented NE–SW and NW–SE due to access difficulties (Fig. 2). We established 5-m radius survey quadrats (n = 198) at 100-m intervals along the transects starting from the midpoint where the nut-cracking site was located. At each quadrat we collected nut-cracking specific and general ecological and vegetation data (further details below).
We collected data digitally. This included georeferencing quadrat mid-points and all data entries (food-providing vegetation, tools, raw materials) using an Arrow Gold GNSS receiver (μHRMS = 2 m; Almeida-Warren et al., 2021; EOS Positioning Systems Inc., 2017). These coordinates were then instantly downloaded via Bluetooth into the GeoGrafi-M application (Mobile GIS Services, 2019) on an android device where we could enter additional data through customized forms.
The data collection period overlapped with two unrelated experimental projects that were being conducted at the “Salon” outdoor laboratory and involved the provisioning of stones and oil palm nuts for chimpanzees to crack. The “Salon” was established in 2009 and is located in an area that, historically, has always experienced a high degree of natural thoroughfare (Matsuzawa, 2011). It is near an active natural nut-cracking site (~ 80 m; Fig. 2) and is at the intersection of several routes which the chimpanzees frequently travel through to access different parts of the forest. These characteristics were targeted specifically to minimize the potential impact on natural behaviour (Matsuzawa, 2011). Thus, while some nut-cracking occurred artificially, we consider the impact on our overall study to be minimal. Nevertheless, we deliberate on some potential implications in the discussion.
Oil palms
For each oil palm encountered during quadrat surveys, we measured the diameter at breast height (DBH) with a diameter tape. For the nut trees associated with nut-cracking sites, we also collected weekly information on new traces of nut-cracking and the availability of edible nuts on the ground. With aid from field assistants, we determined nut edibility by collecting a random sample of nuts within a 2-metre radius of the oil palm to estimate how many contained an edible kernel or were rotten (following Koops et al., 2013). We did not crack open the nuts so as not to affect future availability, but the local people harvest palm fruits and nuts and are able to identify whether or not they are edible (Humle & Matsuzawa, 2004). As per Koops et al. (2013), we scored presence of edible nuts as: (0) nuts absent, (1) 1–50 nuts, (2) 51–100 nuts, and (3) > 100 nuts.
We monitored the nut-cracking sites weekly during the first field season (22 January 2018–03 May 2018) and once at the beginning and end of the second season (weeks of 29 October 2018 and 10 December 2018) to record evidence of nut-cracking activity and availability of oil palm nuts. HDC collected additional monitoring data during the weeks of 30 September 2019, 27 April 2020, and 25 May 2020. However, only the nut-cracking sites documented prior to 22 January (n = 25) were checked for the full 15 weeks.
Tools and raw materials
We recorded all lithic material according to size (the length of the longest axis), raw material type, and portability (whether loose or imbedded in the ground). Adapted from Koops et al. (2013), we scored size into six categories: (1) 1–2 cm, (2) 3–5 cm, (3) 6–10 cm, (4) 11–20 cm, (5) 21–30 cm, and (6) >30 cm. We defined tools and by-products of nut-cracking as stones that showed at least one of the following: a) traces of wear from nut-cracking, b) nutshell remains on or around them, and c) could be refitted with another stone with evidence of a) or b). For this study, the variable Tools included all lithic materials used for nut-cracking except for fragments that could no longer be used for nut-cracking.
We also scored the collective tool assemblage for status of nut-cracking activity, either: (Active) There were new signs of nut-cracking activity during the fieldwork period. Shell fragments or nut powder were visible on top of or around tools, and there was at least one hammer and anvil pair with impact points that lacked signs of chemical or organic weathering (i.e., rust, moss), or (Inactive) There were no signs of recent nut-cracking activity during the entire fieldwork period. Cracked nut kernels were either absent, or present but showed clear signs of decay. Iron oxide or moss had developed on tool impact points.
Vegetation
During quadrat surveys, we recorded all wild plant species known to be consumed by the chimpanzees of Bossou. We then cross-referenced the recorded species with the current list of chimpanzee food resources to identify those which were sources of fruit — the preferred food-type of chimpanzees. We considered trees with a DBH ≥ 5 cm (Hockings et al., 2009), lianas and shrubs with a DBH ≥ 2 cm (Gerwing et al., 2006), and THV of any dimensions. However, while THV is a frequently consumed food item by the chimpanzees of Bossou (Humle, 2011a), it differs from other wild plant foods in that it has a lower calorific value (Bryson-Morrison et al., 2020) and often an individual plant can only be sourced once, after which it is permanently depleted. To distinguish these types of food sources (perennial versus ephemeral), THV was documented as a separate variable. We also encountered a small number of domesticated or crop foods that are eaten by chimpanzees. This largely consisted of cassava tubers (Manihot esculenta) and banana plants (Musa sp.). While crops are frequently consumed by Bossou chimpanzees, especially during the wet season when wild fruit is scarce, use of these areas is conditioned by additional factors such as the high risk of antagonistic interactions with humans (Bryson-Morrison et al., 2017; Hockings et al., 2009). Such species would therefore warrant allocation to a third vegetation category. However, due to the small sample size of cultivated plants (n = 46), we did not include them in the present study.
Nests
Because few nests were documented at the quadrat level or along transects (n = 4), we conducted additional forest-wide surveys, targeting areas that were known nesting locations. When a nest was encountered, we maintained elevation and direction of travel and documented all nests within 50 m of the nest and either side of the projected route until no further nests were visible over a 50 m stretch in any direction (modified from Hernandez-Aguilar, 2009). We recorded nests of all ages, excluding any recent nests that could have been built for daytime resting. Day nests are typically smaller and simpler in construction (Koops et al., 2012), but are more easily identifiably when most of the leaves are still green. We also only surveyed each area once to ensure nests were not recorded multiple times. For data analysis, nests were divided into spatial clusters. Chimpanzees in Bossou typically nest together within 30 m of each other (Humle, 2003). To accommodate variation between multiple nights, we defined a cluster as a 100-m diameter area with a minimum of 21 documented nests, representing a minimum of three sleep-events for the collective chimpanzee population (n = 7) as a proxy for a habitual nesting location. We achieved this in QGIS (Version 2.18.4), by creating a 50-m buffer zone around each nest and using the ‘count points in polygon’ function to identify zones with a minimum of 21 nests. The distance to nearest nest cluster variable used in the following analyses was computed with the ‘distance to nearest hub’ function, and was defined as the distance from a quadrat datum to the centre of the nearest nest cluster.
Watercourses
We used watercourse data collected by HDC in 2008/2009. HDC traced on foot all streams and rivers large enough for small amounts of water to be drawn year-round and recorded them using the track feature on a hand-held GPS device (Garmin Oregon 700; accuracy = ± 3 m). The distance to nearest river variable used in the following analyses was computed in QGIS, and was defined as the distance between a quadrat datum to the nearest point along the mapped watercourses.
Data analysis
Tool site selection
Our initial inspection of the data revealed that no nut-cracking occurred in quadrats where oil palms were absent. This is consistent with previous literature describing that nut-cracking occurs in close proximity to a nut tree (Carvalho et al., 2008). We therefore restricted data analysis to quadrats where an oil palm was present, as the absence of an oil palm would mask the potential effect of the other variables of interest. Additionally, there was very little variation in the number of oil palms in each quadrat, with only 15% of oil palm quadrats documented with more than one oil palm — only two of which were quadrats where nut-cracking was present. For this reason, we also did not include the number of oil palms as a predictor. The final dataset had a total of 82 quadrats, 40 of which had traces of nut-cracking activity. We used a binomial generalized linear model (GLM; Zuur et al., 2009) with a logit link function to investigate, using quadrat data, the effect of five main predictors (raw materials, wild food trees, THV, distance to nearest nest cluster, distance to nearest river) on the presence (1) versus absence (0) of a tool site in a given quadrat. We also assessed the effect of more restricted variables including raw materials within the most common size range for tools (95% of tools. Size class: 3, 4, 5) and wild fruit-providing trees — see Electronic Supplementary Material (ESM1), Table S1. Because these constituted subsets of raw materials and wild food-providing trees respectively, we analysed them under three sub-models (ESM1, Table S2). In the first sub-model we replaced raw materials with the corresponding subset of raw materials with size class 3–5. In the second sub-model we substituted wild food-providing trees with the subset formed only of fruit-providing trees. The third sub-model included raw materials of size class 3–5 and fruit-providing trees instead of raw materials and wild food trees. We compared models using the Akaike’s Information Criterion for small sample sizes (AICc; Burnham et al., 2004) as an indicator of the best model fit (ESM1, Table S4).
Tool site use
From a total of 361 monitoring observations, only 35 cases of recent nut-cracking events were identified for 17 out of the 25 monitored nut-cracking sites, where nut-cracking occurred between 1 and 4 times. Because of single (n = 1) sample sizes for two and four events, frequency of activity was recoded as “Low” (≤ 2 events; n = 10) and “High” (> 2 events; n = 7). The small sample size (n = 17) was too small to justify a generalized linear (mixed) model [GL(M)M]. Thus, we only use descriptive statistics to examine tool site use and potential differences in the local environment, such as nut availability, raw materials, wild food trees, distance to nearest nest cluster, and distance to nearest river.
Tool site inactivity
We used a binomial GLM with ‘logit’ link to investigate the effect of mean nut availability, raw materials, and wild food trees on tool-site inactivity. The response variable included nut-cracking sites that were classified as active (response = 0; n = 24) with those classified as inactive (response = 1; n = 16). The final dataset included 40 tool sites. Akin to the model for tool site selection, we also analysed four additional sub-models (ESM1, Table S5). In the first sub-model we substituted raw materials with the subset for raw materials of size class 3–5. The second sub-model replaced raw materials with tools, which included only the raw materials that were used as tools and remain functional. The third and fourth sub-models were largely identical to the previous two models, with the wild fruit trees subset replacing its parent variable. We compared these models using the AICc as an indicator of best model fit (ESM1, Table S7).
General considerations
We performed all analyses in R Studio (version 1.1.383; R Studio Team, 2016), using R (version 4.1.0; R Core Team, 2021). Threshold for statistical significance was set to p ≤ 0.05. Data exploration for each GLM considered in tool site selection and tool site inactivity, followed the protocol described in Zuur et al. (2010) and did not raise any concerns. We assessed collinearity among the explanatory variables for each model by calculating the variance inflation factors (VIF) using the function ‘vif’ of the ‘car’ package (Jon Fox & Weisberg, 2011). None of the models indicated any multicollinearity issues (Maximum VIF = 1.39, Quinn & Keough, 2002).
We performed significance tests for each of the models considered. To assess the significance of the full models, we ran likelihood ratio tests (LRT) using the ‘anova’ function which compared each model to a corresponding null model from which all fixed effects were excluded (Dobson, 2002). We then tested the significance of main effects for each model by systematically dropping them one at a time and comparing the resulting model with the full model using the ‘drop1’ function (Dobson, 2002). P-values for the individual effects were based on the LRT results from the ‘drop1’ function. We verified model assumptions by plotting residuals versus fitted values and versus each covariate in the model (Zuur & Ieno, 2016). We also assessed influential observations by calculating and plotting the Cook’s distance (Smith & Warren, 2019); all values were under the recommended threshold of 1, suggesting no evidence of influential points (John Fox, 2002; Smith & Warren, 2019). Finally, we calculated the AICc for model comparison using the ‘MuMIn’ package (Bartón, 2020). We focus our discussion on the model that yielded the lowest AICc, with reference to other models where relevant. The full statistical results for these models are provided in the ESM1, Tables S2–S7.
For tool site use, we computed descriptive statistics for high and low frequency nut-cracking activity relative to each variable of interest. This included the minimum, maximum, mean, and standard deviation, except for variables with non-normal distributions for which we report minimum, maximum, median and interquartile range. Normality assumptions were assessed using the Shapiro–Wilk test.
Ethical Note
All tool site and ecological data were collected when chimpanzees were absent from the survey locations. Efforts to ensure minimal disturbance of nut-cracking sites included: keeping all tools in their original locations, not removing or cracking nuts, and collecting stone samples from existing tool fragments whenever possible. To ensure the safety and health of the chimpanzee community, all members of the research team wore surgical masks and maintained a distance of at least 10 m when observing them. Research was conducted in accordance with all the research requirements of the Republic of Guinea, and the ethical protocols set out by The University of Oxford, the Kyoto University Primate Research Institute, and the Institut de Recherche Environmentale de Bossou (IREB).
Data availability
The source code and datasets generated and/or analysed during this study are available at https://doi.org/10.5281/zenodo.6723338.
Results
Tool site selection
The sub-model which included the raw material subset of size class 3–5 was the best fitted model according to the AICc (ESM1, Table S4) and had a clear effect on the probability of a nut-cracking site occurring in a location where at least one oil palm was present (full–null model comparison, LRT: df = 5, deviance = 56.519, p < 0.001). Raw materials had a significant positive effect on tool site prediction, as did food trees, while distance to nest cluster had a significant negative effect (Table I; Fig. 3). All other fixed effects were non-significant (Table I). The sub-model replacing wild food trees with the fruit trees subset yielded the worst model fit, in which fruit trees were not a significant predictor (ESM1 Tables S3–S4).
Probability of encountering a tool site used for nut-cracking by wild chimpanzees in Bossou (Guinea) in response to: a raw materials of size class 3–5, b trees that are sourced by chimpanzees for food, c Distance to the nearest nest cluster. Points represent partial residuals (n = 82), and shaded areas indicate a 95% CI. Lower grey bars indicate the distribution of negative residuals, and upper grey bars indicate the distribution of positive residuals. Data were collected between December 2017 and December 2018.
Tool site use
Over 15 weeks, only 33 cases of nut-cracking were recorded for 17 out of 25 monitored tool sites. At ten of the 17 sites, we recorded one to two nut-cracking events (low frequency), with the remaining seven showing recent traces between three and four times during the monitoring period (high frequency). In general, the number of tools was higher at nut-cracking sites that registered a higher frequency of nut-cracking activity (mean ± SD = 11.429 ± 3.780) than at low frequency sites (mean ± SD = 7.100 ± 3.143; Table II; Fig. 4a). Similar differences were found for raw materials and the raw material subset of size class 3–5, although in both cases two low frequency sites had over 30 stones available, whereas the highest number recorded at high frequency sites was 19 (Table II; Fig. 4b).
Frequency of tool site use by wild chimpanzees in Bossou (Guinea) relative to: a raw materials that have been used as tools, b raw materials of size class 3–5, c mean availability of oil palm nuts, d wild trees that are sourced by chimpanzees for food, e distance to nearest nest cluster (km), f distance to nearest river (km). Boxes illustrate the second and third quartiles, with whiskers indicating the first and fourth quartiles. Solid lines represent median values, and points indicate outliers. Grey circles specify individual points, and means are represented by diamonds. Data were collected over 15 weeks between January 2018 and May 2018.
Mean nut availability ranged between 1.111–2.111 at all sites where more than two nut-cracking events took place. Low frequency sites ranged similarly (min = 0.625, max = 2.000), with only two sites registering a mean nut availability lower than one. With regard to wild food availability, three out of ten low frequency sites did not have wild food trees nearby, including any that could provide wild fruits (Fig. 4d). For all other sites, the number of wild food trees and wild fruit trees varied similarly across low frequency (mean ± SD = 4.00 ± 3.916) and high frequency (mean ± SD = 4.286 ± 2.690) nut-cracking sites (Table II).
On average, high-frequency sites (mean ± SD = 148.714 ± 64.047 m) were approximately 100 m nearer to nesting locations than low-frequency sites (mean ± SD = 245.660 ± 132.760 m; Table II; Fig. 4e), with all high-frequency sites located under 250 m of a nesting site. Conversely, six out of ten of low-frequency sites were located further away, reaching distances of up to 520 m from the nearest nesting location. Distance to the nearest river ranged similarly among low-frequency and high-frequency sites (Table II). All nut-cracking sites were situated between 40–300 m of a water course, and in both low and high frequency categories, six sites were less than 150 m away (Fig. 4f).
Tool site inactivity
Out of the sub-models, the tool subset model yielded the best fit, although the AICc for the tool and fruit trees model was only marginally higher and produced comparable results (ESM1, Tables S6–S7). Comparison of the tool subset model with the null model was significant (LRT: df = 3, deviance = 13.198, p = 0.004). Overall, we found that lower values of mean nut availability and a lower number of tools were both significant predictors of tool site inactivity, while wild food trees had no effect (Fig. 5; Table III). However, the data distributions suggest that the model is not very robust (Fig. 5).
Probability of inactivity of tool sites used by wild chimpanzees in Bossou (Guinea) in response to: a mean nut availability, b raw materials that have been used as tools. Points represent partial residuals for individual quadrats (n = 24) and shaded areas indicate a 95% CI. Lower grey bars indicate the distribution of negative residuals, and upper grey bars indicate the distribution of positive residuals. Data was collected between December 2017 and December 2018.
Discussion
Tool site selection
From the initial inspection of the data, it was evident that at least one oil palm, specifically an oil palm in close proximity (within 10 m), is required for nut-cracking to occur in a given location. Our results further show that the abundance of raw materials and food trees as well as proximity to the nearest nest cluster are also important predictors for whether a tool site is established at an oil-palm location. This suggests that, in addition to the ecological pre-requisites of nut-cracking, i.e., a producing oil palm and raw materials for tools, other perennial resources that form part of the chimpanzee diet (wild food-providing trees), as well as non-food related activities (sleep sites), are influential in the spatial distribution of nut-cracking locations. In contrast, THV had a non-significant effect on whether tool sites occurred. Food trees are replenishable, often seasonally, and constitute reliable resources that can be returned to regularly. The fact that THV did not bear a clear effect on tool site occurrence suggests that ephemeral food sources are not ecological drivers of tool site selection.
Many primate species have goal-orientated foraging trajectories towards spatially permanent resources, and it is probable that they use mental maps to guide their resource exploitation strategies (Trapanese et al., 2019). Our results provide tentative evidence that the chimpanzees of Bossou may behave in a similar way, whereby nut-cracking activities take place within a foraging strategy that primarily targets perennial, high-value food sources (trees), while low-energy foods such as THV act as part of an opportunistic strategy during forage-on-the-go.
Distance to nearest nest cluster was a significant predictor in all models, whereby the likelihood of a tool site occurring increased with proximity to nest locations. Research at other field sites has found that nest sites occur in areas of high food availability (Basabose & Yamagiwa, 2002; Carvalho et al., 2015; Furuichi & Hashimoto, 2004; Goodall, 1962; Janmaat et al., 2014). Given that nut-cracking sites are also located in areas with a greater number of food providing trees, and bearing in mind that the Bossou chimpanzees source oil palms for a range of other resources (e.g., fruit, pith, palm heart) and also nest in their crowns (Humle & Matsuzawa, 2001, 2004; Yamakoshi & Sugiyama, 1995), it is possible that the relationship between nut-cracking sites, proximity to nest sites, and food availability is indicative that these areas are activity hotspots, rich in resources, and with habitat characteristics that are suitable for a range of core chimpanzee activities.
Distance to the nearest river was not a significant predictor in any of the models. This contradicts previous research in the nearby forest of Diécké, that identified that nut-cracking locations occurred near waterlines (Carvalho et al., 2007). Equally, it would seem, at least in the case of Bossou, that water is neither the sole or primary provisioner of stones of tools. Given the proximity of the Bossou and Diécké field sites (approx. 50 km) and their similar climates it is unlikely that this is due to differences in aridity or water availability. However, the chimpanzees of Diécké crack different nut species, Panda oleosa and Coula edulis, which are absent in Bossou and are probably more water-dependent than the oil palm on account of being exclusively rainforest-dwelling species (Burkill, 1985; Moupela et al., 2014). Thus, this discrepancy may be connected to the different plant species exploited and their respective ecology and distribution.
Emerging research on the role and importance of water in shaping primate behaviour, adaptations, and landscape use is providing increasing evidence that there are differences in water-dependence between populations. Rainforest-dwelling apes can usually obtain their daily hydration requirements from the food they consume, and can go several days without drinking (Pontzer et al., 2021). However, for primates that live in predominantly arid habitats, water is a critical resource that shapes movement patterns and landscape use (Barton et al., 1992; Lindshield et al., 2021; Pruetz & Herzog, 2017). Fongoli chimpanzees in Senegal usually drink water at least once a day, and often spend time near water sources during dry months to stay cool (Lindshield et al., 2021; Pruetz & Bertolani, 2009). Conversely, the Bossou forest is much more humid, with a long wet season. Its many streams and small forest area provide a hydrological landscape in which chimpanzees are rarely more than 300 m away from water. Furthermore, during a total of ~500 hours of focal follows, the Bossou chimpanzees were only seen to drink water on eight occasions, suggesting that they can get most of their fluids from the foods they consume, in line with the general trend for non-human apes (Pontzer et al., 2021).
While water was not a significant factor for Bossou, we hypothesize that it could be a major ecological driver regarding the spatial distribution and reuse of tool sites by savannah-living chimpanzees. The Fongoli chimpanzees do not crack nuts, but they engage in termite-fishing, which is also a spatially discrete technological activity tethered to the location of termite mounds (Bogart & Pruetz, 2008, 2011), much like nut-cracking.
Tool site use
On average, the number of functional tools was greater at sites with a higher frequency of nut-cracking events, even though some low-frequency sites had a much greater number of usable stones available overall. This discrepancy could indicate that the visible traces of nut-cracking found on tools act as visual cues for stimulating further nut-cracking behaviour, and potentially also lead to the transport of further raw materials for tools to these locations. The repeated use of discrete locations through stigmergy — the principle that behavioural traces left in the environment can stimulate the occurrence of further activity — has been suggested to have led to the emergence of persistent places during the Middle Pleistocene (Matthew Pope et al., 2006; Matthew Pope, 2017; Shaw et al., 2016). Similar hypotheses featuring local and stimulus enhancement in chimpanzees have also been discussed as processes of social learning (e.g., in the development of technical skills; Musgrave et al., 2020; Tennie et al., 2020; Whiten, 2021), as well as why some plants are sourced more intensively than others for the manufacture of termite-fishing tools (Almeida-Warren et al., 2017). Conversely, it could indicate that chimpanzees prefer sites with material that they are already familiar with. Previous, experimentally-induced research in Bossou has demonstrated that chimpanzees reuse hammer–anvil pairs (tool-sets) more often than others, that there is both group- and individual-level preference for certain tool-sets (S. Carvalho et al., 2009), and that chimpanzees are selective of the types of materials they use for nut-cracking (Carvalho et al., 2008). Analogous studies on chimpanzee plant technologies suggest similar patterns in the selection of materials for termite-fishing, ant-dipping, honey gathering and water extraction (Almeida-Warren et al., 2017; Koops et al., 2015; Lamon et al., 2018; Pascual-Garrido et al., 2012; Pascual-Garrido & Almeida-Warren, 2021).
Distance to nearest nest cluster also showed a noteworthy difference, whereby the frequency of nut-cracking events was marginally greater at tool sites that were closer to nest locations. These results mirror those found for tool-site selection, and offer further tentative support that active tool sites and their frequency of use is influenced by their distribution relative to current activity hotspots.
The number of wild food and fruit trees was largely the same for all active nut-cracking sites. This suggests that while food-providing trees are good indicators of tool-site selection, they may not be good predictors of site use, because the data collected did not capture temporal changes in food availability or frequency of foraging activity. On the other hand, nests are temporary features that, on average, rarely preserve for longer than 6 months in non-savannah environments (Ihobe, 2005; Kamgang et al., 2020; Zamma & Makelele, 2012). Therefore, they are a better spatial proxy for recent ranging patterns and possibly explain why differences were found for nests, but not for vegetation.
Some consideration needs to be given to the low number of weekly traces of nut-cracking events recorded per tool site during the 15 weeks of monitoring. This is partially due to the fact that not all active tool sites were monitored, with a further seven traces found through indirect observations at non-monitored sites. Another contributing factor could be that monitoring largely took place during the dry season when primary foods such as wild fruits are in abundance. Our data indicates that a minimum of 40 nut-cracking events took place at natural nut-cracking sites during the 15-week monitoring period, averaging approximately three events per week, which may be sufficient for the existing chimpanzee population.
Out of the nine nut-cracking events we witnessed during group follows, six took place at the outdoor laboratory (Fig. 2). The fact that tools and edible nuts were artificially guaranteed at this location, contrary to natural nut-cracking sites dependent on oil palm productivity, could explain why a comparatively higher number of nut-cracking events were observed there, similar to patterns recorded by Hockings et al. (2009). There is a possibility, therefore, that the experiments being conducted in the outdoor laboratory may have detracted chimpanzees from cracking nuts elsewhere.
Nevertheless, due to small sample sizes, we would need more data to test these hypotheses, including spatial information of food availability across both dry and wet seasons. For future research, it would be important to investigate over a longer timescale whether and how often chimpanzees visited the parts of the forest where tool sites are located, and the potential effects of the outdoor laboratory on natural behaviour. This could make use of complementary data from camera traps placed in strategic locations, as full-day focal follows are not permitted in Bossou.
Tool site inactivity
Understanding the contexts of tool site inactivity is an important step in investigating the conditions required for nut-cracking to occur and persist over time in a particular location, and the factors that might lead to its abandonment. Our data suggest that mean nut availability, used as a proxy for tree productivity, and a high abundance of tools are important in maintaining the active status of a nut-cracking site. The latter provides further tentative evidence in support of stigmergy. However, there are clear exceptions that appear to not fit the model (Fig. 5), suggesting that other factors that were not considered in the analysis may also be at play.
The Bossou forest suffers from a great deal of human activity, particularly slash-and-burn agriculture, which leads to frequent and rapid changes in the spatial distribution of resources and localized vegetation composition (Hockings, 2011). While oil palms are not cut down during this process and are highly resistant to fire (Yamakoshi, 2011), the changes in the surrounding landscape and the increase in human presence may deter chimpanzees from visiting those areas, especially if they are near the forest boundary. Conversely, cultivated land that contains desirable food items (e.g., banana, mango, papaya) can often attract chimpanzees (Hockings, 2011), and perhaps, under these conditions, the chimpanzees prioritize the prized fruit over nuts that can be found almost anywhere.
Site inactivity could also be an artefact of population decline, whereby fewer resources are sufficient to sustain the entire population. Previous literature has suggested that the Bossou forest has a carrying capacity for around 20 chimpanzees (Sugiyama & Fujita, 2011), so it is possible that the current population may no longer need to depend as highly on nuts to supplement their diets. A future longitudinal comparison drawing from historical and contemporary data will help investigate and test this further.
Perspectives on the formation of hominin technological landscapes
While current evidence suggests that the lithic technologies of early hominins and chimpanzees differ in form and function (Arroyo & de la Torre, 2016; Toth et al., 2006), it is likely they had similar plant-dominated diets supported by invertebrates and sporadic meat consumption (Panger et al., 2002). Thus, it is plausible that, like chimpanzees, early hominin tool use operated within behavioural landscapes influenced by localized environmental parameters, where foraging strategies were shaped by the distribution and availability of predictable food sources, the dietary dependence on extractive foraging, and the availability of the necessary raw materials, as well as by the location of places for sleeping. With the aid of primate archaeological inference, visualizing the spatial distribution of hominin lithic assemblages within this framework will be instrumental in providing crucial insights for reconstructing the patterns of landscape use and resource exploitation.
The present study suggests that the technological landscape of the chimpanzees of Bossou shares affinities with the ‘favoured places’ model (Schick, 1987; Schick & Toth, 1993), which proposed that hominin tool sites formed at the centre of foraging areas where hominins would process and consume food, rest, and socialize, with sites being used more intensively in areas with higher resource abundance. Such ‘activity hotspots’ would have acted as ecological tethers, shaping early hominin movement and foraging patterns, which, in turn, would have led to the formation and repeated use of tool sites over time. As the most conspicuous evidence of these locations, stone tool assemblages may hold important clues for uncovering behaviours beyond those associated to lithic technology, and serve as starting points to search for traces of other activities, such as sleeping, foraging, and insectivory, that are currently extremely rare in the archaeological record.
Although water was not a significant predictor in any of the models in this study, evidence from other chimpanzee sites such as Diécké and Fongoli suggests that the importance of water depends both on the environment’s capacity to meet and maintain the chimpanzees’ physiological needs and on the relevance of water for locating other resources, such as water-dependent nut trees or raw materials for tools. This is likely to have also been the case for early hominins, as evidenced by research that has found that early stone tool sites are spatially linked to water sources, and that this relationship changes in response to environmental shifts as well as anatomical and technological transitions in the hominin record (Rogers et al., 1994). Over time, water-conservation adaptations as well as the development of means to transport water (Pontzer et al., 2021) would have enabled hominins to become less water-depended and range away from permanent water sources. Nevertheless, water may still have played an important role as a provisioner or aquatic resources (Braun et al., 2010), and as a refuge during periods of extreme seasonality and climatic variation (Joordens et al., 2019). Expanding this research to other chimpanzee field sites and exploring water as a foraging context such as for algae- and crab-fishing (Boesch et al., 2017; Koops et al., 2019) will shed light on the diverse contexts in which water may shape landscape-scale patterns of early hominin behaviour and the strength of its influence.
This research draws upon the work of Glynn Isaac (e.g., Isaac, 1981; Isaac et al., 1981; Isaac & Harris, 1980) who pioneered the application of landscape-scale approaches to the study of hominin assemblages, from which the first concrete models of hominin site formation were developed. Further studies will help guide future human origins research and provide an empirical framework for modelling and testing hypotheses of early hominin behaviour associated to the archaeological record of our earliest ancestors.
Conclusions
Our results indicate that proximity to a nut tree, an abundance of raw materials and predictable resources, as well as proximity to a nesting site are important ecological parameters for the establishment of a nut-cracking site in a given location. Sites that attracted a higher frequency of nut-cracking activity were also closer to a nest cluster, which could potentially indicate that nesting sites are important anchors for ranging and activity patterns. Similarly, sites that attracted more nut-cracking activity had a greater number of functional tools. The fact that tool availability was also significantly correlated with tool site inactivity suggests that familiarity of materials used for tools or the visual cues of tool use could be important in the persistence of nut-cracking activities once a site has been established. While nut availability varied similarly among oil palms at active sites, the odds of tool site inactivity were greater when mean nut availability was low, potentially indicating that a decline in oil palm productivity at nut-cracking sites is a driver of site disuse. Together, our results postulate that nut-cracking in Bossou is not only tethered to locations that provide the necessary resources for this activity but is also intimately connected to a broader foraging and behavioural landscape that is mediated by the spatiotemporal availability of primary target resources, such as food-providing trees, as well as the distribution of frequently used nesting locations. These patterns parallel those described by the ‘favoured places’ model proposed for hominin site formation, thus providing a new framework for elucidating early hominin behaviour and landscape use. Preliminary comparisons with other sites regarding the importance of ecological features such as the effect of water on tool use and ranging patterns, suggests that the ecology of chimpanzee technology is context-specific and should be examined with this in mind. Further studies investigating the technological landscapes of other chimpanzee populations, as well as the integration of long-term data, will help better understand the effect of different environmental and demographic contexts on the factors driving the spatial distribution and reuse of both chimpanzee and hominin tool sites, adding further detail to this picture.
References
Abwe, E. E., & Morgan, B. J. (2008). The Ebo forest: four years of preliminary research and conservation of the Nigeria–Cameroon chimpanzee (Pan troglodytes vellerosus). Pan Africa News, 15(2), 26–28.
Almeida-Warren, K., Braun, D. R., & Carvalho, S. (2021). The DistoX2: A methodological solution to archaeological mapping in poorly accessible environments. Journal of Archaeological Science: Reports, 35, 102688. https://doi.org/10.1016/J.JASREP.2020.102688.
Almeida-Warren, K., Sommer, V., Piel, A. K., & Pascual-Garrido, A. (2017). Raw material procurement for termite fishing tools by wild chimpanzees in the Issa valley, Western Tanzania. American Journal of Physical Anthropology, 164(2), 292–304. https://doi.org/10.1002/ajpa.23269.
Arroyo, A., & de la Torre, I. (2016). Assessing the function of pounding tools in the Early Stone Age: A microscopic approach to the analysis of percussive artefacts from Beds I and II, Olduvai Gorge (Tanzania). Journal of Archaeological Science, 74, 23–34. https://doi.org/10.1016/j.jas.2016.08.003.
Badji, L., Ndiaye, P. I., Lindshield, S. M., Ba, C. T., & Pruetz, J. D. (2018). Savanna chimpanzee (Pan troglodytes verus) nesting ecology at Bagnomba (Kedougou, Senegal). Primates, 59(3), 235–241. https://doi.org/10.1007/s10329-017-0647-2.
Barrett, B. J., Monteza-Moreno, C. M., Dogandžić, T., Zwyns, N., Ibáñez, A., & Crofoot, M. C. (2018). Habitual stone-tool-aided extractive foraging in white-faced capuchins. Cebus capucinus. Royal Society: Open Science, 5, 181002. https://doi.org/10.1098/rsos.181002.
Bartón, K. (2020). MuMIn: multi-model Inference. R package version 1.43.17.
Barton, R. A., Whiten, A., Strum, S. C., Byrne, R. W., & Simpson, A. J. (1992). Habitat use and resource availability in baboons. Animal Behaviour, 43(5), 831–844. https://doi.org/10.1016/S0003-3472(05)80206-4.
Basabose, A. K., & Yamagiwa, J. (2002). Factors affecting nesting site choice in chimpanzees at Tshibati, Kahuzi–Biega national park: Influence of sympatric gorillas. International Journal of Primatology, 23(2), 263–282. https://doi.org/10.1023/A:1013879427335.
Bobe, R., Behrensmeyer, A. K., & Chapman, R. E. (2002). Faunal change, environmental variability and late Pliocene hominin evolution. Journal of Human Evolution, 42(4), 475–497. https://doi.org/10.1006/jhev.2001.0535.
Bobe, R., & Carvalho, S. (2019). Hominin diversity and high environmental variability in the Okote Member, Koobi Fora Formation, Kenya. Journal of Human Evolution, 126, 91–105. https://doi.org/10.1016/j.jhevol.2018.10.012.
Boesch, C., & Boesch, H. (1984). Mental map in wild chimpanzees: An analysis of hammer transports for nut cracking. Primates, 25(2), 160–170. https://doi.org/10.1007/BF02382388.
Boesch, C., Bombjaková, D., Boyette, A., & Meier, A. (2017). Technical intelligence and culture: nut cracking in humans and chimpanzees. American Journal of Physical Anthropology, 163(2), 339–355. https://doi.org/10.1002/ajpa.23211.
Boesch, C., Kalan, A. K., Agbor, A., Arandjelovic, M., Dieguez, P., Lapeyre, V., & Kühl, H. S. (2017). Chimpanzees routinely fish for algae with tools during the dry season in Bakoun. Guinea. American Journal of Primatology, 79(3), 1–7. https://doi.org/10.1002/ajp.22613.
Bogart, S. L., & Pruetz, J. D. (2008). Ecological context of savanna chimpanzee (Pan troglodytes verus) termite fishing at Fongoli. Senegal. American Journal of Primatology, 70(6), 605–612. https://doi.org/10.1002/ajp.20530.
Bogart, S. L., & Pruetz, J. D. (2011). Insectivory of savanna chimpanzees (Pan troglodytes verus) at Fongoli. Senegal. American Journal of Physical Anthropology, 145(1), 11–20. https://doi.org/10.1002/ajpa.21452.
Braun, D. R., Harris, J. W. K., Levin, N. E., McCoy, J. T., Herries, A. I. R., Bamford, M. K., et al (2010). Early hominin diet included diverse terrestrial and aquatic animals 1.95 Ma in East Turkana, Kenya. Proceedings of the National Academy of Sciences of the United States of America, 107(22), 10002–10007. https://doi.org/10.1073/pnas.1002181107.
Bryson-Morrison, N., Beer, A., Gaspard Soumah, A., Matsuzawa, T., & Humle, T. (2020). The macronutrient composition of wild and cultivated plant foods of West African chimpanzees (Pan troglodytes verus) inhabiting an anthropogenic landscape. American Journal of Primatology, 82(3), e23102. https://doi.org/10.1002/ajp.23102.
Bryson-Morrison, N., Tzanopoulos, J., Matsuzawa, T., & Humle, T. (2017). Activity and habitat use of chimpanzees (Pan troglodytes verus) in the anthropogenic landscape of Bossou, Guinea, West Africa. International Journal of Primatology, 38(2), 282–302. https://doi.org/10.1007/s10764-016-9947-4.
Burkill, H. M. (1985). Panda oleosa Pierre [family PANDACEAE]. In The useful plants of west tropical Africa (Vol 4). Royal Botanic Gardens, Kew: University Press of Virginia.
Burnham, K. P., & Anderson, D. R. (Eds.). (2004). Model selection and multimodel inference (2nd ed.). New York, NY: Springer. https://doi.org/10.1007/b97636
Carvalho, J. S., Meyer, C. F. J., Vicente, L., & Marques, T. A. (2015). Where to nest? Ecological determinants of chimpanzee nest abundance and distribution at the habitat and tree species scale. American Journal of Primatology, 77(2), 186–199. https://doi.org/10.1002/ajp.22321.
Carvalho, S. (2011). Diécké Forest, Guinea: Delving into Chimpanzee Behavior Using Stone Tool Surveys. In T Matsuzawa, T. Humle, & Y. Sugiyama (Eds.), The Chimpanzees of Bossou and Nimba (pp. 301–312). Tokyo: Springer Japan.
Carvalho, S., & Almeida-Warren, K. (2019). Primate Archaeology. In J. Chun Choe (Ed.), Encyclopedia of Animal Behavior (2nd ed., Vol. 1, pp. 397–407). Elsevier. https://doi.org/10.1016/B978-0-12-809633-8.90156-0
Carvalho, S., Biro, D., McGrew, W. C., & Matsuzawa, T. (2009). Tool–composite reuse in wild chimpanzees (Pan troglodytes): archaeologically invisible steps in the technological evolution of early hominins? Animal Cognition, 12(S1), 103–114. https://doi.org/10.1007/s10071-009-0271-7.
Carvalho, S., Cunha, E., Sousa, C., & Matsuzawa, T. (2008). Chaînes opératoires and resource-exploitation strategies in chimpanzee (Pan troglodytes) nut cracking. Journal of Human Evolution, 55(1), 148–163. https://doi.org/10.1016/j.jhevol.2008.02.005.
Carvalho, S., Cunha, E., Sousa, C., & Matsuzawa, T. (2011). Extensive surveys of chimpanzee stone tools: from the telescope to the magnifying glass. In Tetsuro Matsuzawa, T. Humle, & Y. Sugiyama (Eds.), The Chimpanzees of Bossou and Nimba (pp. 145–155). Tokyo: Springer Japan.
Carvalho, S., Matsuzawa, T., & McGrew, W. C. (2013). From pounding to knapping: How chimpanzees can help us to model hominin lithics. In C. M. Sanz, J. Call, & C. Boesch (Eds.), Tool Use in Animals (pp. 225–241). Cambridge University Press. https://doi.org/10.1017/CBO9780511894800.015.
Carvalho, S., & McGrew, W. C. (2012). The origins of the Oldowan: Why chimpanzees (Pan troglodytes) still are good models for technological evolution in Africa. In M. Domínguez-Rodrigo (Ed.), Stone Tools and Fossil Bones: Debates in the Archaeology of Human Origins (pp. 201–221). Cambridge University Press. https://doi.org/10.1017/CBO9781139149327.010.
Carvalho, S., Sousa, C., & Matsuzawa, T. (2007). New nut-cracking sites in Diecké Forest, Guinea: an overview of the surveys. Pan Africa News, 14(1), 11–13.
Dobson, A. J. (2002). An introduction to generalized linear models. Chapman & Hall/CRC.
EOS Positioning Systems Inc. (2017). Arrow Gold. https://eos-gnss.com/wp-content/uploads/2017/11/eos-arrow-gold-revC-v4.pdf. Accessed 12 March 2020
Falótico, T., Proffitt, T., Ottoni, E. B., Staff, R. A., & Haslam, M. (2019). Three thousand years of wild capuchin stone tool use. Nature Ecology & Evolution, 3(7), 1034–1038. https://doi.org/10.1038/s41559-019-0904-4.
Fox, John. (2002). An R and S-Plus companion to applied regression. Sage Publications.
Fox, J., & Weisberg, S. (2011). An R companion to applied regression (2nd ed.). Sage.
Furuichi, T., & Hashimoto, C. (2004). Botanical and topographical factors influencing nesting-site selection by chimpanzees in Kalinzu Forest, Uganda. International Journal of Primatology, 25(4), 755–765. https://doi.org/10.1023/B:IJOP.0000029121.25284.7F.
Gerwing, J. J., Schnitzer, S. A., Burnham, R. J., Bongers, F., Chave, J., Dewalt, S. J., et al (2006). A standard protocol for liana censuses. Biotropica, 38(2), 256–261. https://doi.org/10.1111/j.1744-7429.2006.00134.x.
Goodall, J. (1962). Nest building behavior in the free ranging chimpanzee. Annals of the New York Academy of Sciences, 102(2), 455–467. https://doi.org/10.1111/j.1749-6632.1962.tb13652.x.
Goodall, J. (1963). Feeding behaviour of wild chimpanzees: a preliminary report. Symposium of the Zoological Society of London, 10, 39–48.
Gruber, T., Potts, K. B., Krupenye, C., Byrne, M. R., Mackworth-Young, C., McGrew, W. C., et al (2012). The influence of ecology on chimpanzee (Pan troglodytes) cultural behavior: a case study of five Ugandan chimpanzee communities. Journal of Comparative Psychology, 126(4), 446–457. https://doi.org/10.1037/a0028702.
Grund, C., Neumann, C., Zuberbühler, K., & Gruber, T. (2019). Necessity creates opportunities for chimpanzee tool use. Behavioral Ecology, 30(4), 1136–1144. https://doi.org/10.1093/beheco/arz062.
Gumert, M. D., Kluck, M., & Malaivijitnond, S. (2009). The physical characteristics and usage patterns of stone axe and pounding hammers used by long-tailed macaques in the Andaman Sea Region of Thailand. American Journal of Primatology, 608(71), 594–608. https://doi.org/10.1002/ajp.20694.
Hernandez-Aguilar, R. A. (2009). Chimpanzee nest distribution and site reuse in a dry habitat: implications for early hominin ranging. Journal of Human Evolution, 57(4), 350–364. https://doi.org/10.1016/j.jhevol.2009.03.007.
Hernandez-Aguilar, R. A., Moore, J., & Stanford, C. B. (2013). Chimpanzee nesting patterns in savanna habitat: Environmental influences and preferences. American Journal of Primatology, 75(10), 979–994. https://doi.org/10.1002/ajp.22163.
Hockings, K. J. (2011). The crop-raiders of the sacred hill. In T. Matsuzawa, T. Humle, & Y. Sugiyama (Eds.), The chimpanzees of Bossou and Nimba (pp. 211–220). Springer Japan.
Hockings, K. J., Anderson, J. R., & Matsuzawa, T. (2006). Road crossing in chimpanzees: a risky business. Current Biology, 16(17), R668–R670. https://doi.org/10.1016/j.cub.2006.08.019.
Hockings, K. J., Anderson, J. R., & Matsuzawa, T. (2009). Use of wild and cultivated foods by chimpanzees at Bossou, Republic of Guinea: feeding dynamics in a human-influenced environment. American Journal of Primatology, 71(8), 636–646. https://doi.org/10.1002/ajp.20698.
Hockings, K. J., Anderson, J. R., & Matsuzawa, T. (2012). Socioecological adaptations by chimpanzees, Pan troglodytes verus, inhabiting an anthropogenically impacted habitat. Animal Behaviour, 83(3), 801–810. https://doi.org/10.1016/j.anbehav.2012.01.002.
Hockings, K. J., McLennan, M. R., Carvalho, S., Ancrenaz, M., Bobe, R., Byrne, R. W., et al (2015). Apes in the Anthropocene: flexibility and survival. Trends in Ecology and Evolution, 30(4), 215–222. https://doi.org/10.1016/j.tree.2015.02.002.
Humle, T. (2003). Culture and variation in wild chimpanzee behaviour: a study of three communities in West Africa. PhD Thesis. University of Stirling.
Humle, T. (2011a). Location and Ecology. In T. Matsuzawa, T. Humle, & Y. Sugiyama (Eds.), The chimpanzees of Bossou and Nimba (pp. 13–22). Tokyo: Springer Japan.
Humle, T. (2011b). The 2003 epidemic of a flu-like respiratory disease at Bossou. In T. Matsuzawa, T. Humle, & Y. Sugiyama (Eds.), The chimpanzees of Bossou and Nimba (pp. 325–333). Springer Japan.
Humle, T., & Matsuzawa, T. (2001). Behavioural diversity among the wild chimpanzee populations of Bossou and neighbouring areas, Guinea and Côte d’Ivoire. West Africa. Folia Primatologica, 72(2), 57–68. https://doi.org/10.1159/000049924.
Humle, T., & Matsuzawa, T. (2004). Oil palm use by adjacent communities of chimpanzees at Bossou and Nimba Mountains, West Africa. International Journal of Primatology, 25(3), 551–581. https://doi.org/10.1023/B:IJOP.0000023575.93644.f4.
Humle, T., & Matsuzawa, T. (2009). Laterality in hand use across four tool-use behaviors among the wild chimpanzees of Bossou, Guinea. West Africa. American Journal of Primatology, 71(1), 40–48. https://doi.org/10.1002/ajp.20616.
Ihobe, H. (2005). Life span of chimpanzee beds at the Mahale Mountains National Park. Tanzania. Pan Africa News, 12(1), 10–12.
Isaac, G. L. (1981). Stone Age visiting cards: approaches to the study of early land use patterns. In I. Hodder, G. L. Isaac, & N. Hammond (Eds.), Pattern of the past: studies in honour of David Clarke (pp. 131–155). Cambridge University Press.
Isaac, G. L., & Harris, J. W. K. (1980). Proceedings of the 8th Panafrican Congress on Prehistory and Quaternary Studies. In R. E. F. Leakey & B. A. Ogot (Eds.), A method for determining the characteristics of artefacts between sites in the Upper Member of the Koobi Fora Formation, East Lake Turkana (pp. 19–22). International Louis Leakey Memorial Institute for African Prehistory.
Isaac, G. L., Harris, J. W. K., & Marshall, F. (1981). Small is informative: the application of the study of mini-sites and least effort criteria in the interpretation of the Early Pleistocene archaeological record at Koobi Fora, Kenya. In B. Isaac (Ed.), The Archaeology of human origins: papers by Glynn Isaac (pp. 101–119). Cambridge University Press.
Janmaat, K. R. L., Polansky, L., Ban, S. D., & Boesch, C. (2014). Wild chimpanzees plan their breakfast time, type, and location. Proceedings of the National Academy of Sciences of the United States of America, 111(46), 16343–16348. https://doi.org/10.1073/pnas.1407524111.
Joordens, J. C. A., Feibel, C. S., Vonhof, H. B., Schulp, A. S., & Kroon, D. (2019). Relevance of the eastern African coastal forest for early hominin biogeography. Journal of Human Evolution, 131, 176–202. https://doi.org/10.1016/J.JHEVOL.2019.03.012.
Kalan, A. K., Kulik, L., Arandjelovic, M., Boesch, C., Haas, F., Dieguez, P., et al (2020). Environmental variability supports chimpanzee behavioural diversity. Nature Communications, 11(1), 4451. https://doi.org/10.1038/s41467-020-18176-3.
Kamgang, S. A., Carme, T. C., Bobo, K. S., Abwe, E. E., Gonder, M. K., & Sinsin, B. (2020). Assessment of in situ nest decay rate for chimpanzees (Pan troglodytes ellioti Matschie, 1914) in Mbam-Djerem National Park, Cameroon: implications for long-term monitoring. Primates, 61(2), 189–200. https://doi.org/10.1007/s10329-019-00768-3.
Koops, K., McGrew, W. C., de Vries, H., & Matsuzawa, T. (2012). Nest-building by chimpanzees (Pan troglodytes verus) at Seringbara, Nimba Mountains: antipredation, thermoregulation, and antivector hypotheses. International Journal of Primatology, 33(2), 356–380. https://doi.org/10.1007/s10764-012-9585-4.
Koops, K., McGrew, W. C., & Matsuzawa, T. (2013). Ecology of culture: do environmental factors influence foraging tool use in wild chimpanzees, Pan troglodytes verus? Animal Behaviour, 85(1), 175–185. https://doi.org/10.1016/j.anbehav.2012.10.022.
Koops, K., Schöning, C., Isaji, M., & Hashimoto, C. (2015). Cultural differences in ant-dipping tool length between neighbouring chimpanzee communities at Kalinzu, Uganda. Scientific Reports, 5(1), 12456. https://doi.org/10.1038/srep12456.
Koops, K., Visalberghi, E., & van Schaik, C. P. (2014). The ecology of primate material culture. Biology Letters, 10(11), 20140508. https://doi.org/10.1098/rsbl.2014.0508.
Koops, K., Wrangham, R. W., Cumberlidge, N., Fitzgerald, M. A., Van Leeuwen, K. L., Rothman, J. M., & Matsuzawa, T. (2019). Crab-fishing by chimpanzees in the Nimba Mountains, Guinea. Journal of Human Evolution, 133, 230–241. https://doi.org/10.1016/J.JHEVOL.2019.05.002.
Kühl, H. S., Boesch, C., Kulik, L., Haas, F., Arandjelovic, M., Dieguez, P., et al (2019). Human impact erodes chimpanzee behavioral diversity. Science, 363(6434), 1453–1455. https://doi.org/10.1126/science.aau4532.
Lamon, N., Neumann, C., Gier, J., Zuberbühler, K., & Gruber, T. (2018). Wild chimpanzees select tool material based on efficiency and knowledge. Proceedings of the Royal Society B: Biological Sciences, 285(1888), 20181715. https://doi.org/10.1098/rspb.2018.1715.
Langergraber, K. E., Prüfer, K., Rowney, C., Boesch, C., Crockford, C., Fawcett, K., et al (2012). Generation times in wild chimpanzees and gorillas suggest earlier divergence times in great ape and human evolution. Proceedings of the National Academy of Sciences, 109(39), 15716–15721. https://doi.org/10.1073/pnas.1211740109.
Lindshield, S., Hernandez-Aguilar, R. A., Korstjens, A. H., Marchant, L. F., Narat, V., Ndiaye, P. I., et al (2021). Chimpanzees (Pan troglodytes) in savanna landscapes. Evolutionary Anthropology: Issues, News, and Reviews, 30(6), 399–420. https://doi.org/10.1002/EVAN.21924.
Luncz, L. V., Proffitt, T., Kulik, L., Haslam, M., & Wittig, R. M. (2016). Distance–decay effect in stone tool transport by wild chimpanzees. Proceedings of the Royal Society B: Biological Sciences, 283(1845), 20161607. https://doi.org/10.1098/rspb.2016.1607.
Matsuzawa, T. (2011). Field experiments of tool-use. In T. Matsuzawa, T. Humle, & Y. Sugiyama (Eds.), The chimpanzees of Bossou and Nimba (pp. 157–164). Springer Japan.
McCarthy, M. S., Lester, J. D., & Stanford, C. B. (2017). Chimpanzees (Pan troglodytes) flexibly use introduced species for nesting and bark feeding in a human-dominated habitat. International Journal of Primatology, 38(2), 321–337. https://doi.org/10.1007/s10764-016-9916-y.
McGrew, W. C. (2010). In search of the last common ancestor: new findings on wild chimpanzees. Philosophical Transactions of the Royal Society B: Biological Sciences, 365(1556), 3267–3276. https://doi.org/10.1098/rstb.2010.0067.
McGrew, W. C. (2021). Sheltering chimpanzees. Primates, 62(3), 445–455. https://doi.org/10.1007/s10329-021-00903-z.
Mercader, J., Barton, H., Gillespie, J., Harris, J., Kuhn, S., Tyler, R., & Boesch, C. (2007). 4,300-year-old chimpanzee sites and the origins of percussive stone technology. Proceedings of the National Academy of Sciences of the United States of America, 104(9), 3043–3048. https://doi.org/10.1073/pnas.0607909104.
Mobile GIS Services. (2019). GeoGrafi-M — MGISS. https://mgiss.co.uk/product/geografi-m/. Accessed 20 September 2019
Morgan, D. B., & Sanz, C. M. (2006). Chimpanzee feeding ecology and comparisons with sympatric gorillas in the Goualougo Triangle, Republic of Congo. In G. Hohmann, M. M. Robbins, & C. Boesch (Eds.), Feeding ecology in apes and other primates (pp. 97–122). Cambridge University Press.
Moupela, C., Doucet, J.-L., Daïnou, K., Brostaux, Y., Fayolle, A., & Vermeulen, C. (2014). Reproductive ecology of Coula edulis Baill., source of a valuable non-timber forest product. Tropical Ecology, 55(3), 327–338.
Musgrave, S., Lonsdorf, E., Morgan, D., Prestipino, M., Bernstein-Kurtycz, L., Mundry, R., & Sanz, C. M. (2020). Teaching varies with task complexity in wild chimpanzees. Proceedings of the National Academy of Sciences of the United States of America, 117(2), 969–976. https://doi.org/10.1073/pnas.1907476116.
Ndiaye, P. I., Badji, L., Lindshield, S. M., & Pruetz, J. D. (2018). Nest-building behaviour by chimpanzees (Pan troglodytes verus) in the non-protected area of Diaguiri (Kedougou, Senegal): implications for conservation. Folia Primatologica, 89(5), 316–326. https://doi.org/10.1159/000490945.
Ohashi, G. (2015). Pestle-pounding and nut-cracking by wild chimpanzees at Kpala. Liberia. Primates, 56(2), 113–117. https://doi.org/10.1007/s10329-015-0459-1.
Ottoni, E. B., & Izar, P. (2008). Capuchin monkey tool use: Overview and implications. Evolutionary Anthropology, 17(4), 171–178. https://doi.org/10.1002/evan.20185.
Panger, M. A., Brooks, A. S., Richmond, B. G., & Wood, B. (2002). Older than the Oldowan? Rethinking the emergence of hominin tool use. Evolutionary Anthropology, 11(6), 235–245. https://doi.org/10.1002/evan.10094.
Pascual-Garrido, A. (2018). Scars on plants sourced for termite fishing tools by chimpanzees: Towards an archaeology of the perishable. American Journal of Primatology, 80(9), e22921. https://doi.org/10.1002/ajp.22921.
Pascual-Garrido, A., & Almeida-Warren, K. (2021). Archaeology of the perishable. Current Anthropology, 62(3), 333–362. https://doi.org/10.1086/713766.
Pascual-Garrido, A., Buba, U., Nodza, G., & Sommer, V. (2012). Obtaining raw material: plants as tool sources for Nigerian chimpanzees. Folia Primatologica, 83(1), 24–44. https://doi.org/10.1159/000338898.
Pontzer, H., Brown, M. H., Wood, B. M., Raichlen, D. A., Mabulla, A. Z. P., Harris, J. A., et al (2021). Evolution of water conservation in humans. Current Biology, 31, 1–7. https://doi.org/10.1016/j.cub.2021.02.045.
Pope, M. (2017). Thresholds in behaviour, thresholds of visibility: landscape processes, asymmetries in landscape records and niche construction in the formation of the Palaeolithic record. In M. Pope, C. Gamble, & J. McNabb (Eds.), crossing the Human Threshold Dynamic Transformation and Persistent Places During the Late Middle Pleistocene. Routledge.
Pope, M., Russel, K., & Watson, K. (2006). Biface form and structured behaviour in the Acheulean. Lithics, 27, 44–57.
Popkin, B. M., D’Anci, K. E., & Rosenberg, I. H. (2010). Water, hydration, and health. Nutrition Reviews, 68(8), 439–458. https://doi.org/10.1111/J.1753-4887.2010.00304.X.
Potts, K. B., Baken, E., Levang, A., & Watts, D. P. (2016). Ecological factors influencing habitat use by chimpanzees at Ngogo, Kibale National Park. Uganda. American Journal of Primatology, 78(4), 432–440. https://doi.org/10.1002/AJP.22513.
Potts, R. (1998). Environmental hypotheses of hominin evolution. American Journal of Physical Anthropology, 107, 93–136. https://doi.org/10.1002/(SICI)1096-8644(1998)107:27+<93::AID-AJPA5>3.0.CO;2-X.
Potts, R., Dommain, R., Moerman, J. W., Behrensmeyer, A. K., Deino, A. L., Riedl, S., et al (2020). Increased ecological resource variability during a critical transition in hominin evolution. Science. Advances, 6(43), eabc8975. https://doi.org/10.1126/sciadv.abc8975.
Pruetz, J. D., & Bertolani, P. (2009). Chimpanzee (Pan troglodytes verus) Behavioral responses to stresses associated with living in a savannah–mosaic environment: implications for hominin adaptations to open habitats. PaleoAnthropology, 2009, 252–262. https://doi.org/10.4207/pa.2009.art33.
Pruetz, J. D., & Herzog, N. M. (2017). Savanna chimpanzees at Fongoli, Senegal, navigate a fire landscape. Current Anthropology, 58(16), S337–S350. https://doi.org/10.1086/692112.
Quinn, G. P., & Keough, M. J. (2002). Experimental design and data analysis for biologists. Cambridge University Press.
R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing.
R Studio Team. (2016). RStudio: Integrated Development for R. Boston, MA: RStudio, Inc.
Reed, K. E. (1997). Early hominid evolution and ecological change through the African Plio-Pleistocene. Journal of Human Evolution, 32(2–3), 289–322. https://doi.org/10.1006/jhev.1996.0106.
Robbins, M. M., & Hohmann, G. (2006). Primate feeding ecology: an integrative approach. In G. Hohmann, M. M. Robbins, & C. Boesch (Eds.), Feeding ecology in apes and other primates (pp. 1–13). Cambridge University Press.
Rogers, M. J., Harris, J. W. K., & Feibel, C. S. (1994). Changing patterns of land use by Plio-Pleistocene hominids in the Lake Turkana Basin. Journal of Human Evolution, 27(1–3), 139–158. https://doi.org/10.1006/jhev.1994.1039.
Rolian, C., & Carvalho, S. (2017). Tool use and manufacture in the last common ancestor of Pan and Homo. In M. Muller, R. Wrangham, & D. Pilbeam (Eds.), Chimpanzees and human evolution (pp. 602–644). Belknap Press of Harvard University Press.
Sanz, C. M., & Morgan, D. B. (2013). Ecological and social correlates of chimpanzee tool use. Philosophical Transactions of the Royal Society B: Biological Sciences, 368(1630), 20120416. https://doi.org/10.1098/rstb.2012.0416.
Schick, K. D. (1987). Modeling the formation of Early Stone Age artifact concentrations. Journal of Human Evolution, 16(7–8), 789–807. https://doi.org/10.1016/0047-2484(87)90024-8.
Schick, K. D., & Toth, N. (1993). Making silent stones speak. Simon and Schuster.
Sept, J. M. (1992). Was there no place like home?: A new perspective on early hominid archaeological sites from the mapping of chimpanzee nests. Current Anthropology, 33(2), 187–207. https://doi.org/10.1086/204050.
Shaw, A., Bates, M., Conneller, C., Gamble, C., Julien, M.-A., McNabb, J., et al (2016). The archaeology of persistent places: the Palaeolithic case of La Cotte de St Brelade, Jersey. Antiquity, 90(354), 1437–1453. https://doi.org/10.15184/aqy.2016.212.
Smith, C., & Warren, M. (2019). GLMs in R for Ecology. Amazon Publishing.
Stewart, F. A., Piel, A. K., & McGrew, W. C. (2011). Living archaeology: artefacts of specific nest site fidelity in wild chimpanzees. Journal of Human Evolution, 61(4), 388–395. https://doi.org/10.1016/j.jhevol.2011.05.005.
Strier, K. B. (2011). Primate Behavioral Ecology (4th ed.). Allyn and Bacon.
Sugiyama, Y. (2004). Demographic parameters and life history of chimpanzees at Bossou. Guinea. American Journal of Physical Anthropology, 124(2), 154–165. https://doi.org/10.1002/ajpa.10345.
Sugiyama, Y., & Fujita, S. (2011). The demography and reproductive parameters of Bossou chimpanzees. In T. Matsuzawa, T. Humle, & Y. Sugiyama (Eds.), The chimpanzees of Bossou and Nimba (pp. 23–34). Springer Japan.
Sugiyama, Y., & Koman, J. (1979). Tool-using and -making behavior in wild chimpanzees at Bossou. Guinea. Primates, 20(4), 513–524. https://doi.org/10.1007/BF02373433.
Tennie, C., Bandini, E., van Schaik, C. P., & Hopper, L. M. (2020). The zone of latent solutions and its relevance to understanding ape cultures. Biology and Philosophy, 35(5), 55. https://doi.org/10.1007/s10539-020-09769-9.
Toth, N., Schick, K. D., & Semaw, S. (2006). A comparative study of the stone tool-making skills of Pan, Australopithecus, and Homo sapiens. In N. Toth & K. Schick (Eds.), The Oldowan: case studies into the earliest Stone Age (pp. 155–222). Stone Age Institute Press.
Trapanese, C., Meunier, H., & Masi, S. (2019). What, where and when: spatial foraging decisions in primates. Biological Reviews, 94(2), 483–502. https://doi.org/10.1111/brv.12462.
UNEP-WCMC, & IUCN. (2019). Protected planet: The World Database on Protected Areas (WDPA). www.protectedplanet.net. Accessed 9 November 2019
Wessling, E. G., Deschner, T., Mundry, R., Pruetz, J. D., Wittig, R. M., & Kühl, H. S. (2018a). Seasonal variation in physiology challenges the notion of chimpanzees (Pan troglodytes verus) as a forest-adapted species. Frontiers in Ecology and Evolution, 6, 60. https://doi.org/10.3389/fevo.2018.00060.
Wessling, E. G., Kühl, H. S., Mundry, R., Deschner, T., & Pruetz, J. D. (2018b). The costs of living at the edge: Seasonal stress in wild savanna-dwelling chimpanzees. Journal of Human Evolution, 121, 1–11. https://doi.org/10.1016/j.jhevol.2018.03.001.
Whiten, A. (2021). The burgeoning reach of animal culture. Science, 372(6537), eabe6514. https://doi.org/10.1126/science.abe6514.
Whiten, A., Goodall, J., McGrew, W. C., Nishida, T., Reynolds, V., Sugiyama, Y., et al (1999). Cultures in chimpanzees. Nature, 399(6737), 682–685. https://doi.org/10.1038/21415.
Wrangham, R. W. (1977). Feeding behaviour of chimpanzees in Gombe National Park, Tanzania. In T. H. Clutton-Brock (Ed.), Primate ecology: studies of feeding and ranging behavior in lemurs, monkey and apes (pp. 503–538). Academic Press. https://doi.org/10.1016/B978-0-12-176850-8.50022-6.
Yamakoshi, G. (1998). Dietary responses to fruit scarcity of wild chimpanzees at Bossou, Guinea: Possible implications for ecological importance of tool use. American Journal of Physical Anthropology, 106(3), 283–295. https://doi.org/10.1002/(SICI)1096-8644(199807)106:3<283::AID-AJPA2>3.0.CO;2-O.
Yamakoshi, G. (2011). Pestle-pounding behavior: the key to the coexistence of humans and chimpanzees. In T. Matsuzawa, T. Humle, & Y. Sugiyama (Eds.), The chimpanzees of Bossou and Nimba (pp. 107–115). Springer Japan.
Yamakoshi, G., & Sugiyama, Y. (1995). Pestle-pounding behavior of wild chimpanzees at Bossou, Guinea: a newly observed tool-using behavior. Primates, 36, 489–500. https://doi.org/10.1007/BF02382871.
Zamma, K., & Makelele, M. (2012). Comparison of the longevity of chimpanzee beds between two areas in the Mahale Mountains National Park. Tanzania. Pan Africa News, 19(2), 25–28. https://doi.org/10.5134/168176.
Zuur, A. F., & Ieno, E. N. (2016). A protocol for conducting and presenting results of regression-type analyses. Methods in Ecology and Evolution, 7(6), 636–645. https://doi.org/10.1111/2041-210X.12577.
Zuur, A. F., Ieno, E. N., & Elphick, C. S. (2010). A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution, 1(1), 3–14. https://doi.org/10.1111/j.2041-210X.2009.00001.x.
Zuur, A. F., Ieno, E. N., Walker, N. J., Saveliev, A. A., & Smith, G. M. (2009). GLM and GAM for absence–presence and proportional data. In Mixed Effects Models and Extensions in Ecology with R (pp. 245–259). : Springer. https://doi.org/10.1007/978-0-387-87458-6_10
Acknowledgements
We are grateful to Vincent Mamy, Lawe Goigbe, Boniface Zogbila, Gouanou Zogbila, Jules Doré, and Pascal Goumi for support in the field; Dr. Jesse van der Grient and Dr. Alexander Mielke for statistical advice; Dr. Dora Biro and Dr. Bronwyn Tarr for helpful feedback on the original manuscript; Dr. Kat Koops and one anonymous reviewer for their constructive comments on the submitted manuscript; Dr. Tatyana Humle and Prof. William McGrew for feedback on subsequent revisions. We also thank the Direction Nationale de la Recherche Scientifique, the Institut de Recherche Environmentale de Bossou (Guinea), and the Kyoto University Primate Research Institute, for research permissions and logistical support. KAW was funded by the Fundação pela Ciência e Tecnologia (grant number: SFRH/BD/115085/2016), supported by the Programa Operacional Capital Humano (POCH) and the European Union; the Boise Trust Fund (University of Oxford); and the National Geographic Society (grant number: EC-399R-18); TM thanks the Japan Society for the Promotion of Science (JSPS) Leading Graduate Program (U04-PWS), JSPS core-to-core CCSN, and the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT)/JSPS-KAKENHI (grant numbers: #07102010, #12002009, #16002001, #20002001, #24000001, #16H06283); SC thanks the Leverhulme Trust (grant number: PLP 2016-114) which supported part of the field equipment and logistics.
Author information
Authors and Affiliations
Contributions
KAW is the main author and contributor and was responsible for conceptualization, methodology, primary data collection, formal analysis, interpretation, data curation, visualization and writing of the original manuscript. HDC collected data, participated in methodological and logistical preparations while in the field, and commented on the manuscript. TM provided resources, data, methodological and logistic advice, and manuscript revisions. SC contributed towards conceptualization, methodology and revisions of the manuscript, and provided supervision, resources, and data. All authors gave final approval for publication and agree to be held accountable for the work performed therein.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Handling Editor: Joanna Setchell
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Almeida-Warren, K., Camara, H.D., Matsuzawa, T. et al. Landscaping the Behavioural Ecology of Primate Stone Tool Use. Int J Primatol 43, 885–912 (2022). https://doi.org/10.1007/s10764-022-00305-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10764-022-00305-y