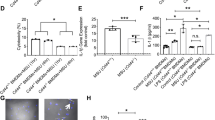
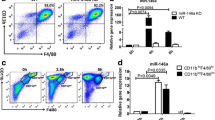
Abstract
Gout is a self-limiting form of inflammatory arthropathy caused by the formation of urate crystals due to hyperuricemia. The resolution of gout involves the transition of proinflammatory M1-type macrophages to anti-inflammatory M2-type macrophages, as well as neutrophil-mediated extracellular trap (NET) formation. However, the underlying mechanisms of these changes are not clear. Studies have confirmed that high expression of CD39 on macrophages and neutrophils can trigger the polarization of macrophages from a proinflammatory state to an anti-inflammatory state. Recent studies have shown that the pathogenesis of gout involves extracellular ATP (eATP), and the synergistic effect of MSU and extracellular ATP can cause gout. CD39 is a kind of ATP hydrolysis enzyme that can degrade eATP, suggesting that CD39 may inhibit the aggravation of inflammation in gout and participate in the remission mechanism of gout. To confirm this hypothesis, using data mining and flow cytometry, we first found that CD39 expression was significantly upregulated on CD14 + monocytes and neutrophils in gout patients during the acute phase. Inhibition of CD39 by lentivirus or a CD39 inhibitor in acute gout models aggravated gouty arthritis and delayed gout remission. Apyrase, a functional analog of CD39, can significantly reduce the inflammatory response and promote gout remission in acute gout model mice. Our findings confirm that the upregulation of CD39 during gout flare-ups promotes spontaneous remission of acute gouty inflammation.






Similar content being viewed by others
Data Availability
The data presented in this study are available upon request from the corresponding author.
References
Dalbeth, N., A.L. Gosling, A. Gaffo, and A. Abhishek. 2021. Gout. The Lancet 397(10287): 1843–1855.
Campion, E.W., R.J. Glynn, and L.O. Delabry. 1987. Asymptomatic hyperuricemia. Risks and consequences in the normative aging study. The American Journal of Medicine 82(3): 421–426.
Martinon, F., V. Pétrilli, A. Mayor, A. Tardivel, and J. Tschopp. 2006. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440 (7081): 237–241.
Martinon, F., K. Burns, and J. Tschopp. 2002. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Molecular Cell 10 (2): 417–426.
Zahid, A., B. Li, A.J.K. Kombe, T. Jin, and J. Tao. 2019. Pharmacological inhibitors of the NLRP3 inflammasome. Frontiers in Immunology 10: 2538.
Wallace, S.L., H. Robinson, A.T. Masi, J.L. Decker, D.J. McCarty, and T.F. Yü. 1977. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis and Rheumatism 20 (3): 895–900.
Czegley, C., M. Biermann, D. Weidner, M. Hoffmann, M. Herrmann, and C. Schauer. 2014. Monocytes and granulocytes orchestrate induction and resolution of inflammation in gout. 1:88–93.
Martin, W.J., M. Walton, and J. Harper. 2009. Resident macrophages initiating and driving inflammation in a monosodium urate monohydrate crystal-induced murine peritoneal model of acute gout. Arthritis and Rheumatism 60 (1): 281–289.
Scott, P., H. Ma, S. Viriyakosol, R. Terkeltaub, and R. Liu-Bryan. 2006. Engagement of CD14 mediates the inflammatory potential of monosodium urate crystals. Journal of Immunology (Baltimore, Md : 1950) 177(9): 6370–6378.
Liu, L., L. Zhu, M. Liu, L. Zhao, Y. Yu, Y. Xue, et al. 2022. Recent insights into the role of macrophages in acute gout. Frontiers in Immunology 13: 955806.
Zhao, L., W. Ye, Y. Zhu, F. Chen, Q. Wang, X. Lv, et al. 2022. Distinct macrophage polarization in acute and chronic gout. Laboratory Investigation; A Journal of Technical Methods and Pathology 102(10): 1054–1063.
Jeong, J.H., S. Hong, O.C. Kwon, B. Ghang, I. Hwang, Y.G. Kim, et al. 2017. CD14(+) Cells with the phenotype of infiltrated monocytes consist of distinct populations characterized by anti-inflammatory as well as pro-inflammatory activity in gouty arthritis. Frontiers in Immunology 8: 1260.
Maueröder, C., D. Kienhöfer, J. Hahn, C. Schauer, B. Manger, G. Schett, et al. 2015. How neutrophil extracellular traps orchestrate the local immune response in gout. Journal of Molecular Medicine (Berlin, Germany) 93 (7): 727–734.
Landis, R.C., D.R. Yagnik, O. Florey, P. Philippidis, V. Emons, J.C. Mason, et al. 2002. Safe disposal of inflammatory monosodium urate monohydrate crystals by differentiated macrophages. Arthritis and Rheumatism 46 (11): 3026–3033.
Zhang WZ. 2021. Why does hyperuricemia not necessarily induce gout? Biomolecules 11(2).
Dai, X.J., X. Fang, Y. Xia, M.Y. Li, X.M. Li, Y.P. Wang, et al. 2022. ATP-activated P2X7R promote the attack of acute gouty arthritis in rats through activating NLRP3 inflammasome and inflammatory cytokine production. Journal of Inflammation Research 15: 1237–1248.
Li, X., A. Wan, Y. Liu, M. Li, Z. Zhu, C. Luo, et al. 2023. P2X7R mediates the synergistic effect of ATP and MSU crystals to induce acute gouty arthritis. Oxidative Medicine and Cellular Longevity 2023: 3317307.
Tao, J.H., M. Cheng, J.P. Tang, X.J. Dai, Y. Zhang, X.P. Li, et al. 2017. Single nucleotide polymorphisms associated with P2X7R function regulate the onset of gouty arthritis. PLoS ONE 12 (8): e0181685.
Antonioli, L., P. Pacher, E.S. Vizi, and G. Hasko. 2013. CD39 and CD73 in immunity and inflammation. Trends in Molecular Medicine 19 (6): 355–367.
Schetinger, M.R., V.M. Morsch, C.D. Bonan, and A.T. Wyse. 2007. NTPDase and 5’-nucleotidase activities in physiological and disease conditions: New perspectives for human health. BioFactors (Oxford, England) 31 (2): 77–98.
Deaglio, S., and S.C. Robson. 2011. Ectonucleotidases as regulators of purinergic signaling in thrombosis, inflammation, and immunity. Advances in Pharmacology (San Diego, Calif) 61:301–332.
Savio, L.E.B., P. de Andrade Mello, S. Santos, J.C. de Sousa, S.D.C. Oliveira, R.D. Minshall, et al. 2020. P2X7 receptor activation increases expression of caveolin-1 and formation of macrophage lipid rafts, thereby boosting CD39 activity. Journal fo Cell Science 133(5).
Tao, J., and M. Cheng. 2017. Single-nucleotide polymorphisms associated with P2x7r function regulate the onset of gouty arthritis. Annals of the Rheumatic Diseases 76: 769–769.
Zhao, H., C. Bo, Y. Kang, and H. Li. 2017. What else can CD39 tell us? Frontiers in Immunology 8.
Csoka, B., Z.H. Nemeth, G. Toro, B. Koscso, E. Kokai, S.C. Robson, et al. 2015. CD39 improves survival in microbial sepsis by attenuating systemic inflammation. FASEB journal : Official publication of the Federation of American Societies for Experimental Biology 29 (1): 25–36.
Savio, L.E.B., Mello P. de Andrade, V.R. Figliuolo, T.F. de Avelar Almeida, P.T. Santana, S.D.S. Oliveira, et al. 2017. CD39 limits P2X7 receptor inflammatory signaling and attenuates sepsis-induced liver injury. Journal of Hepatology 67 (4): 716–726.
Cohen, H.B., K.T. Briggs, J.P. Marino, K. Ravid, S.C. Robson, and D.M. Mosser. 2013. TLR stimulation initiates a CD39-based autoregulatory mechanism that limits macrophage inflammatory responses. Blood 122 (11): 1935–1945.
Chen, Y., Y. Bao, J. Zhang, T. Woehrle, Y. Sumi, S. Ledderose, et al. 2015. Inhibition of neutrophils by hypertonic saline involves pannexin-1, CD39, CD73, and other ectonucleotidases. Shock (Augusta, Ga) 44 (3): 221–227.
Neogi, T., T.L. Jansen, N. Dalbeth, J. Fransen, H.R. Schumacher, D. Berendsen, et al. 2015. 2015 Gout classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis & Rhematology 67 (10): 2557–2568.
Coderre, T.J., and P.D. Wall. 1987. Ankle joint urate arthritis (AJUA) in rats: an alternative animal model of arthritis to that produced by Freund’s adjuvant. PAIN 28(3).
Eltzschig, H.K., T. Eckle, A. Mager, N. Kuper, C. Karcher, T. Weissmuller, et al. 2006. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circulation Research 99 (10): 1100–1108.
Liu, Y.H., Y.C. Chang, L.K. Chen, P.A. Su, W.C. Ko, Y.S. Tsai, et al. 2019. Corrigendum: The ATP-P2X(7) signaling axis is an essential sentinel for intracellular clostridium difficile pathogen-induced inflammasome activation. Frontiers in Cellular and Infection Microbiology 9: 260.
North, R.A. 2002. Molecular physiology of P2X receptors. Physiological Reviews 82 (4): 1013–1067.
Eltzschig, H.K., D. Kohler, T. Eckle, T. Kong, S.C. Robson, and S.P. Colgan. 2009. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood 113 (1): 224–232.
Eltzschig, H.K., J.C. Ibla, G.T. Furuta, M.O. Leonard, K.A. Jacobson, K. Enjyoji, et al. 2003. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: Role of ectonucleotidases and adenosine A2B receptors. Journal of Experimental Medicine 198 (5): 783–796.
Kuhny, M., T. Hochdorfer, C.K. Ayata, M. Idzko, M. and Huber. 2014. CD39 is a negative regulator of P2X7-mediated inflammatory cell death in mast cells. Cell Communication and Signaling 12.
Zanin, R.F., E. Braganhol, L.S. Bergamin, L.F. Campesato, A.Z. Filho, J.C. Moreira, et al. 2012. Differential macrophage activation alters the expression profile of NTPDase and ecto-5’-nucleotidase. PLoS ONE 7 (2): e31205.
Eltzschig, H.K., L.F. Thompson, J. Karhausen, R.J. Cotta, J.C. Ibla, S.C. Robson, et al. 2004. Endogenous adenosine produced during hypoxia attenuates neutrophil accumulation: Coordination by extracellular nucleotide metabolism. Blood 104 (13): 3986–3992.
Kukulski, F., F. Bahrami, F. Ben Yebdri, J. Lecka, M. Martin-Satue, S.A. Levesque, et al. 2011. NTPDase1 controls IL-8 production by human neutrophils. Journal of immunology (Baltimore, Md:1950) 187(2): 644–653.
Corriden, R., Y. Chen, Y. Inoue, G. Beldi, S.C. Robson, P.A. Insel, et al. 2008. Ecto-nucleoside triphosphate diphosphohydrolase 1 (E-NTPDase1/CD39) regulates neutrophil chemotaxis by hydrolyzing released ATP to adenosine. The Journal of Biological Chemistry 283 (42): 28480–28486.
Reutershan, J., I. Vollmer, S. Stark, R. Wagner, K.C. Ngamsri, and H.K. Eltzschig. 2009. Adenosine and inflammation: CD39 and CD73 are critical mediators in LPS-induced PMN trafficking into the lungs. FASEB journal : Official publication of the Federation of American Societies for Experimental Biology 23 (2): 473–482.
Eltzschig, H.K., T. Weissmüller, A. Mager, and T. Eckle. 2006. Nucleotide metabolism and cell-cell interactions. Methods in Molecular Biology (Clifton, NJ) 341:73–87.
Liu, L., Y. Xue, Y. Zhu, D. Xuan, X. Yang, M. Liang, et al. 2016. Interleukin 37 limits monosodium urate crystal-induced innate immune responses in human and murine models of gout. Arthritis Research & Therapy 18 (1): 268.
Schauer, C., C. Janko, L.E. Munoz, Y. Zhao, D. Kienhofer, B. Frey, et al. 2014. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nature Medicine 20 (5): 511–517.
Chen, Y.-H., S.-C. Hsieh, W.-Y. Chen, K.-J. Li, C.-H. Wu, P.-C. Wu, et al. 2011. Spontaneous resolution of acute gouty arthritis is associated with rapid induction of the anti-inflammatory factors TGFβ1, IL-10 and soluble TNF receptors and the intracellular cytokine negative regulators CIS and SOCS3. Annals of the Rheumatic Diseases 70 (9): 1655.
Sun, X., Y. Wu, W. Gao, K. Enjyoji, E. Csizmadia, C.E. Müller, et al. 2010. CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology 139 (3): 1030–1040.
Deaglio, S., K.M. Dwyer, W. Gao, D. Friedman, A. Usheva, A. Erat, et al. 2007. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. Journal of Experimental Medicine 204 (6): 1257–1265.
Kaczmarek, E., K. Koziak, J. Sevigny, J.B. Siegel, J. Anrather, A.R. Beaudoin, et al. 1996. Identification and characterization of CD39/vascular ATP diphosphohydrolase. The Journal of Biological Chemistry 271 (51): 33116–33122.
Chen, C., X. Li, C. Li, J. Jin, D. Wang, Y. Zhao, et al. 2020. CD39(+) Regulatory T cells attenuate lipopolysaccharide-induced acute lung injury via autophagy and the ERK/FOS pathway. Frontiers in Immunology 11: 602605.
Dai, X.J., J.H. Tao, X. Fang, Y. Xia, X.M. Li, Y.P. Wang, et al. 2018. Changes of Treg/Th17 ratio in spleen of acute gouty arthritis rat induced by MSU crystals. Inflammation 41 (5): 1955–1964.
Mercier, N., T.O. Kiviniemi, A. Saraste, M. Miiluniemi, J. Silvola, S. Jalkanen, et al. 2012. Impaired ATP-induced coronary blood flow and diminished aortic NTPDase activity precede lesion formation in apolipoprotein E-deficient mice. American Journal of Pathology 180 (1): 419–428.
Robson, S.C., E. Kaczmarek, J.B. Siegel, D. Candinas, K. Koziak, M. Millan, et al. 1997. Loss of ATP diphosphohydrolase activity with endothelial cell activation. Journal of Experimental Medicine 185 (1): 153–163.
Yegutkin, G.G., M. Helenius, E. Kaczmarek, N. Burns, S. Jalkanen, K. Stenmark, et al. 2011. Chronic hypoxia impairs extracellular nucleotide metabolism and barrier function in pulmonary artery vasa vasorum endothelial cells. Angiogenesis 14 (4): 503–513.
Keystone EC, Wang MM, Layton M, Hollis S, McInnes IB, Team DCS. 2012. Clinical evaluation of the efficacy of the P2X7 purinergic receptor antagonist AZD9056 on the signs and symptoms of rheumatoid arthritis in patients with active disease despite treatment with methotrexate or sulphasalazine. Annals of the Rheumatic Diseases 71 (10): 1630–1635.
Eser, A., J.F. Colombel, P. Rutgeerts, S. Vermeire, H. Vogelsang, M. Braddock, et al. 2015. Safety and efficacy of an oral inhibitor of the purinergic receptor P2X7 in adult patients with moderately to severely active Crohn’s disease: A randomized placebo-controlled, double-blind, phase IIa study. Inflammatory Bowel Diseases 21 (10): 2247–2253.
Acknowledgements
We thank Yujie Tang, Hongliang Zhang, and Yuting Li for their technical help.
Funding
This work was supported by the Fundamental Research Funds for the Central Universities (YD9110002017).
Author information
Authors and Affiliations
Contributions
JT and CL contributed to the conception and design of the study. CL, XL, YL, JG and HS conducted the experiments and analyzed the data. CL drafted the manuscript. All authors contributed to manuscript revision and approved the submitted version.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Ethics Approval
Our protocols for the collection and processing of animal and human samples were approved by the Animal Ethics Committee (2021-N(A)-041) and Medical Research Ethics Committee (2021 KY No.162) of the First Hospital of the University of Science and Technology of China (Anhui Provincial Hospital), respectively. All persons gave their informed consent before their inclusion in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Luo, C., Liu, X., Liu, Y. et al. Upregulation of CD39 During Gout Attacks Promotes Spontaneous Remission of Acute Gouty Inflammation. Inflammation 47, 664–677 (2024). https://doi.org/10.1007/s10753-023-01936-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-023-01936-w