Abstract
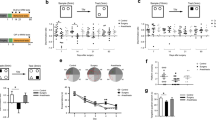
Postoperative cognitive dysfunction (POCD) is a common complication after surgical anesthesia, mainly manifested as memory impairment, decreased attention, and cognitive function with mood and personality changes. Activated microglia (M1-type microglia) have been demonstrated to release inflammatory substances (IL-1β, TNF-α, etc.) that cause neuronal degeneration and death by activating the NF-κB signaling pathway and upregulating Caspase-3 and Bax. However, the pathogenesis of POCD is still not fully understood and needs further research. In the present study, we investigated the effect of M1-type microglia-derived extracellular vesicles (EVsM1−Microglia) in the pathological process of POCD. The levels of NF-κB phosphorylation and IL-1β protein expression in hippocampal neurons were significantly increased in the Surgery group, while PSD95 and MAP2 were significantly decreased. Surgery induced microglia activation, synapse-associated protein decrease, and neuronal degeneration in hippocampus. And the amount of spine and mushroom spine significantly decreased in surgical mice, which was reverted in the presence of IL-1R1 siRNA. In addition, EVsM1−Microglia promoted synaptic loss and neuron degeneration independent of surgery and microglia activation. Furthermore, EVsM1−Microglia promoted memory defects in surgical mice. We demonstrated that EVsM1−Microglia with high expression of IL-1R1 promote POCD development by regulating neuronal inflammation.








Similar content being viewed by others
Availability of Data and Materials
Data and materials relevant to the study are available from the corresponding author upon reasonable request.
References
Humeidan, M.L., J.-P.C. Reyes, A. Mavarez-Martinez, C. Roeth, C.M. Nguyen, E. Sheridan, A. Zuleta-Alarcon, A. Otey, M. Abdel-Rasoul, and S.D. Bergese. 2021. Effect of cognitive prehabilitation on the incidence of postoperative delirium among older adults undergoing major noncardiac surgery: The neurobics randomized clinical trial. JAMA Surgery 156: 148–156. https://doi.org/10.1001/jamasurg.2020.4371.
Patel, A.B.U., V. Weber, A.V. Gourine, and G.L. Ackland. 2022. The potential for autonomic neuromodulation to reduce perioperative complications and pain: A systematic review and meta-analysis. British Journal of Anaesthesia 128: 135–149. https://doi.org/10.1016/j.bja.2021.08.037.
Leslie, M. 2017. The post-op brain. Science 356: 898–900. https://doi.org/10.1126/science.356.6341.898.
Karalapillai, D., L. Weinberg, P. Peyton, L. Ellard, R. Hu, B. Pearce, C.O. Tan, et al. 2020. Effect of intraoperative low tidal volume vs conventional tidal volume on postoperative pulmonary complications in patients undergoing major surgery. JAMA 324: 1–11. https://doi.org/10.1001/jama.2020.12866.
Deiner, S., X. Luo, H.-M. Lin, D.I. Sessler, L. Saager, F.E. Sieber, H.B. Lee, et al. 2017. Intraoperative infusion of dexmedetomidine for prevention of postoperative delirium and cognitive dysfunction in elderly patients undergoing major elective noncardiac surgery. JAMA Surgery 152: e171505. https://doi.org/10.1001/jamasurg.2017.1505.
Deiner, S., X. Liu, H.-M. Lin, R. Jacoby, J. Kim, M. G. Baxter, F. Sieber, K. Boockvar, and M. Sano. 2021. Does postoperative cognitive decline result in new disability after surgery? Annals of Surgery 274: e1108–e1114. https://doi.org/10.1097/SLA.0000000000003764.
Hu, D., R.P. Flick, M.J. Zaccariello, R.C. Colligan, S.K. Katusic, D.R. Schroeder, A.C. Hanson, et al. 2017. Association between exposure of young children to procedures requiring general anesthesia and learning and behavioral outcomes in a population-based birth cohort. Anesthesiology 127: 227–240. https://doi.org/10.1097/ALN.0000000000001735.
Zhang, J., S. Zhu, P. Jin, Y. H., Q. Dai, Q. Zhu, P. Wei, et al. 2020. Graphene oxide improves postoperative cognitive dysfunction by maximally alleviating amyloid beta burden in mice. Theranostics 10: 11908–11920. https://doi.org/10.7150/thno.50616.
Xu, H., L. Chen, X. Zhang, X. Jiang, W. Tian, W. Yu, X. Wang, J. Tian, and D. Su. 2019. Central cholinergic neuronal degeneration promotes the development of postoperative cognitive dysfunction. Laboratory Investigation; a Journal of Technical Methods and Pathology 99: 1078–1088. https://doi.org/10.1038/s41374-018-0174-9.
Wang, T., H. Zhu, Y. Hou, W. Gu, H. Wu, Y. Luan, C. Xiao, and C. Zhou. 2019. Galantamine reversed early postoperative cognitive deficit via alleviating inflammation and enhancing synaptic transmission in mouse hippocampus. European Journal of Pharmacology 846: 63–72. https://doi.org/10.1016/j.ejphar.2018.12.034.
Muhammad, T., M. Ikram, R. Ullah, S.U. Rehman, and M.O. Kim. 2019. Hesperetin, a citrus flavonoid, attenuates LPS-induced neuroinflammation, apoptosis and memory impairments by modulating TLR4/NF-κB signaling. Nutrients 11: E648. https://doi.org/10.3390/nu11030648.
Tong, L.G. A. Prieto, and C.W. Cotman 2018. IL-1β suppresses cLTP-induced surface expression of GluA1 and actin polymerization via ceramide-mediated Src activation. Journal of Neuroinflammation 15: 127. https://doi.org/10.1186/s12974-018-1158-9.
Taoro-Gonzalez, L., Y.M. Arenas, A. Cabrera-Pastor, and V. Felipo 2018. Hyperammonemia alters membrane expression of GluA1 and GluA2 subunits of AMPA receptors in hippocampus by enhancing activation of the IL-1 receptor: Underlying mechanisms. Journal of Neuroinflammation 15: 36. https://doi.org/10.1186/s12974-018-1082-z.
Richter, M., P. Vader, and G. Fuhrmann. 2021. Approaches to surface engineering of extracellular vesicles. Advanced Drug Delivery Reviews 173: 416–426. https://doi.org/10.1016/j.addr.2021.03.020.
Zinger, A., C. Cvetkovic, M. Sushnitha, T. Naoi, G. Baudo, M. Anderson, A. Shetty, et al. 2021. Humanized biomimetic nanovesicles for neuron targeting. Advanced Science (Weinheim, Baden-Wurttemberg, Germany) 8: e2101437. https://doi.org/10.1002/advs.202101437.
Qiu, L.-L., M.-H. Ji, H. Zhang, J.-J. Yang, Xi.-R. Sun, H. Tang, J. Wang, W.-X. Liu, and J.-J. Yang. 2016. NADPH oxidase 2-derived reactive oxygen species in the hippocampus might contribute to microglial activation in postoperative cognitive dysfunction in aged mice. Brain, Behavior, and Immunity 51: 109–118. https://doi.org/10.1016/j.bbi.2015.08.002.
Qi, Z., Q. Xue, H. Wang, B. Cao, Y. Su, Q. Xing, and J.-J. Yang. 2021. Serum CD203c+ extracellular vesicle serves as a novel diagnostic and prognostic biomarker for succinylated gelatin induced perioperative hypersensitive reaction. Frontiers in Immunology 12: 732209. https://doi.org/10.3389/fimmu.2021.732209.
Wu, H., J. Zheng, S. Xu, Y. Fang, Y. Wu, J. Zeng, A. Shao, et al. 2021. Mer regulates microglial/macrophage M1/M2 polarization and alleviates neuroinflammation following traumatic brain injury. Journal of Neuroinflammation 18: 2. https://doi.org/10.1186/s12974-020-02041-7.
Feng, X., M. Valdearcos, Y. Uchida, D. Lutrin, M. Maze, and S.K. Koliwad. 2017. Microglia mediate postoperative hippocampal inflammation and cognitive decline in mice. JCI insight 2: e91229. https://doi.org/10.1172/jci.insight.91229.
Ayata, P., A. Badimon, H.J. Strasburger, M.K. Duff, S.E. Montgomery, Y.-H.E. Loh, A. Ebert, et al. 2018. Epigenetic regulation of brain region-specific microglia clearance activity. Nature neuroscience 21: 1049–1060. https://doi.org/10.1038/s41593-018-0192-3.
Tagge, C.A., A.M. Fisher, O.V. Minaeva, A. Gaudreau-Balderrama, J.A. Moncaster, X-L. Zhang, M.W. Wojnarowicz, et al. 2018. Concussion, microvascular injury, and early tauopathy in young athletes after impact head injury and an impact concussion mouse model. Brain 141: 422–458. https://doi.org/10.1093/brain/awx350.
Salvador, A.F., K. Alves de Lima, and J. Kipnis. 2021. Neuromodulation by the immune system: A focus on cytokines. Nature Reviews. Immunology 21: 526–541. https://doi.org/10.1038/s41577-021-00508-z.
Niu, Haichen, Qian Wang, Weiguang Zhao, Jianxin Liu, Deguang Wang, Bilal Muhammad, Xiaoyu Liu, et al. 2020. IL-1β/IL-1R1 signaling induced by intranasal lipopolysaccharide infusion regulates alpha-Synuclein pathology in the olfactory bulb, substantia nigra and striatum. Brain Pathology (Zurich, Switzerland) 30: 1102–1118. https://doi.org/10.1111/bpa.12886.
Huang, S., X. Ge, J. Yu, Z. Han, Z. Yin, Y. Li, F. Chen, H. Wang, J. Zhang, and P. Lei. 2018. Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB journal: Official publication of the Federation of American Societies for Experimental Biology 32: 512–528. https://doi.org/10.1096/fj.201700673R.
Long, X., X. Yao, Q. Jiang, Y. Yang, X. He, W. Tian, K. Zhao, and H. Zhang. 2020. Astrocyte-derived exosomes enriched with miR-873a-5p inhibit neuroinflammation via microglia phenotype modulation after traumatic brain injury. Journal of Neuroinflammation 17: 89. https://doi.org/10.1186/s12974-020-01761-0.
Clayton, K., J.C. Delpech, S. Herron, N. Iwahara, M. Ericsson, T. Saito, T.C. Saido, S. Ikezu, and T. Ikezu. 2021. Plaque associated microglia hyper-secrete extracellular vesicles and accelerate tau propagation in a humanized APP mouse model. Molecular Neurodegeneration 16: 18. https://doi.org/10.1186/s13024-021-00440-9.
Zhang, R., Y. Fu, M. Cheng, W. Ma, N. Zheng, Y. Wang, and Z. Wu. 2022. sEVsRVG selectively delivers antiviral siRNA to fetus brain, inhibits ZIKV infection and mitigates ZIKV-induced microcephaly in mouse model. Molecular Therapy: The Journal of the American Society of Gene Therapy 30: 2078–2091. https://doi.org/10.1016/j.ymthe.2021.10.009.
Funding
This study was supported by the National Natural Science Foundation of China (81971020).
Author information
Authors and Affiliations
Contributions
ZQ contributed to sample collection, designed the methods, designed experiments design, and performed validation, formal analysis, investigation, data curation, and writing (original draft, review, and editing); YY and YS designed the methods, isolated EVs, and performed NTA analysis; BC and HS: western blot and animal experiments; JJY: conceptualization, writing (review and editing), project administration, and funding acquisition. All the authors have proofread the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qi, Z., Yu, Y., Su, Y. et al. M1-Type Microglia-Derived Extracellular Vesicles Overexpressing IL-1R1 Promote Postoperative Cognitive Dysfunction by Regulating Neuronal Inflammation. Inflammation 46, 2254–2269 (2023). https://doi.org/10.1007/s10753-023-01875-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-023-01875-6