Abstract
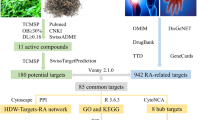
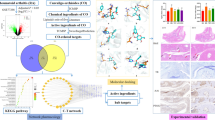
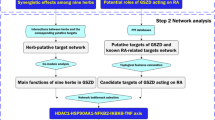
Bavachinin (BVC) is a natural small molecule from the Chinese herb Fructus Psoraleae. It exhibits numerous pharmacological effects, including anti-cancer, anti-inflammation, anti-oxidation, anti-bacterial, anti-viral, and immunomodulatory properties. BVC may serve as a novel drug candidate for the treatment of rheumatoid arthritis (RA). Nevertheless, the effects and mechanisms of BVC against RA are still unknown. BVC targets were selected by Swiss Target Prediction and the PharmMapper database. RA-related targets were collected from the GeneCards, OMIM, DrugBank, TTD, and DisGeNET databases. PPI network construction and enrichment analysis were conducted by taking the intersection target of BVC targets and RA-related targets. Hub targets were further screened using Cytoscape and molecular docking. MH7A cell lines and collagen‐induced arthritis (CIA) mice were used to confirm the preventive effect of BVC on RA and its potential mechanism. Fifty-six RA-related targets of BVC were identified through databases. These genes were primarily enriched in PI3K/AKT signaling pathway according to KEGG enrichment analysis. Molecular docking analysis suggested that BVC had the highest binding energy with PPARG. The qPCR and western blotting results showed that BVC promoted the expression of PPARG at both the mRNA level and protein level. Western blotting indicated that BVC might affect MH7A cell functions through the PI3K/AKT pathway. Furthermore, treatment with BVC inhibited the proliferation, migration, and production of inflammatory cytokines in MH7A cells and induced cell apoptosis to a certain extent. In vivo, BVC alleviated joint injury and inflammatory response in CIA mice. This study revealed that BVC may inhibit the proliferation, migration, and production of inflammatory cytokines in MH7A cells, as well as cell apoptosis through the PPARG/PI3K/AKT signaling pathway. These findings provide a theoretical foundation for RA therapy.








Similar content being viewed by others
Data Availability
The datasets and materials used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- RA:
-
Rheumatoid arthritis
- BVC:
-
Bavachinin
- FLSs:
-
Fibroblast-like synoviocytes
- TCM:
-
Traditional Chinese medicines
- CIA:
-
Collagen‐induced arthritis
- PPARG:
-
Peroxisome proliferator-activated receptor-gamma
- TNF-α:
-
Tumor necrosis factor alpha
- IL‐1β:
-
Interleukin 1β
- IL‐6:
-
Interleukin 6
- IL-10:
-
Interleukin 10
- MMP2:
-
Matrix metalloproteinas 2
- PI3K:
-
Phosphatidylinositol 3-kinase
- p-PI3K:
-
Phospho-PI3K
- AKT:
-
Protein kinase B
- p-AKT:
-
Phospho‐AKT
- CytC:
-
Cytochrome C
- Bcl-2:
-
Reactive oxygen species B‐cell lymphoma 2
- Bax:
-
Bcl2‐associated X protein
- Bad:
-
Bcl2-associated agonist of cell death
- ESR1:
-
Estrogen receptor alpha
- MAPK1:
-
mitogen-activated protein kinase
- SERPINE1:
-
Plasminogen activator inhibitor-1
References
McInnes, I., and G. Schett. 2011. The pathogenesis of rheumatoid arthritis. The New England journal of medicine. 365 (23): 2205–2219. https://doi.org/10.1056/NEJMra1004965.
Singh, J., D. Furst, A. Bharat, et al. 2012. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis care & research. 64 (5): 625–639. https://doi.org/10.1002/acr.21641.
Smolen, J., R. Landewé, F. Breedveld, et al. 2014. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Annals of the Rheumatic Diseases 73 (3): 492–509. https://doi.org/10.1136/annrheumdis-2013-204573.
Lee, D., and M. Weinblatt. 2001. Rheumatoid arthritis. Lancet (London, England). 358 (9285): 903–911. https://doi.org/10.1016/s0140-6736(01)06075-5.
Xu, H., S. Zheng, and D. Fox. 2020. Editorial: Immunomodulatory functions of fibroblast-like synoviocytes in joint inflammation and destruction during rheumatoid arthritis. Frontiers in Immunology 11: 955. https://doi.org/10.3389/fimmu.2020.00955.
Bottini, N., and G. Firestein. 2013. Duality of fibroblast-like synoviocytes in RA: Passive responders and imprinted aggressors. Nature Reviews Rheumatology 9 (1): 24–33. https://doi.org/10.1038/nrrheum.2012.190.
Falconer, J., A. Murphy, S. Young, et al. 2018. Review: Synovial cell metabolism and chronic inflammation in rheumatoid arthritis. Arthritis & Rhematology 70 (7): 984–999. https://doi.org/10.1002/art.40504.
Samarpita, S., R. Ganesan, and M. Rasool. 2020. Cyanidin prevents the hyperproliferative potential of fibroblast-like synoviocytes and disease progression via targeting IL-17A cytokine signalling in rheumatoid arthritis. Toxicology and Applied Pharmacology 391: 114917. https://doi.org/10.1016/j.taap.2020.114917.
Ma, J.-D., J. Jing, J.W. Wang, et al. 2019. A novel function of artesunate on inhibiting migration and invasion of fibroblast-like synoviocytes from rheumatoid arthritis patients. Arthritis Research and Therapy 21 (1). https://doi.org/10.1186/s13075-019-1935-6.
Gan, D., W. Cheng, L. Ke, et al. 2021. Taraxasterol suppresses inflammation in IL-1beta-induced rheumatoid arthritis fibroblast-like synoviocytes and rheumatoid arthritis progression in mice. Frontier Pharmacology 12: 631891. https://doi.org/10.3389/fphar.2021.631891.
Meng, M., Z. Yue, L. Chang, et al. 2021. Anti-rheumatoid arthritic effects of Paris saponin VII in human rheumatoid arthritis fibroblast-like synoviocytes and adjuvant-induced arthritis in rats. Frontier Pharmacology 12: 683698. https://doi.org/10.3389/fphar.2021.683698.
Commission, C.P. 2015. Chinese Pharmacopoeia (2015, VolumeI), 187. Beijing: Chinese Medicine Science and Technology Press.
Zhou, Y.-T., L. Zhu, Y. Yuan, et al. 2020. Effects and mechanisms of five Psoralea prenylflavonoids on aging-related diseases. Oxidative Medicine and Cellular Longevity. 2020: 2128513. https://doi.org/10.1155/2020/2128513.
Chen, Q., Y. Li, and Z. Chen. 2012. Separation, identification, and quantification of active constituents in by high-performance liquid chromatography with UV, ion trap mass spectrometry, and electrochemical detection. J Pharm Anal. 2 (2): 143–151. https://doi.org/10.1016/j.jpha.2011.11.005.
Chen, X., Y. Yang, and Y. Zhang. 2013. Isobavachalcone and bavachinin from Psoraleae Fructus modulate Aβ42 aggregation process through different mechanisms in vitro. FEBS Letters. 587 (18): 2930–2935. https://doi.org/10.1016/j.febslet.2013.07.037.
Hung, S.-Y., S.-C. Lin, S. Wang, et al. 2021. Bavachinin induces G2/M cell cycle arrest and apoptosis via the ATM/ATR signaling pathway in human small cell lung cancer and shows an antitumor effect in the xenograft model. Journal of Agricultural and Food Chemistry. 69 (22): 6260–6270. https://doi.org/10.1021/acs.jafc.1c01657.
Matsuda, H., S. Kiyohara, S. Sugimoto, et al. 2009. Bioactive constituents from Chinese natural medicines. XXXIII. Inhibitors from the seeds of Psoralea corylifolia on production of nitric oxide in lipopolysaccharide-activated macrophages. Biological and Pharmaceutical Bulletin 32 (1): 147–149.
Nepal, M., H.J. Choi, B.-Y. Choi, et al. 2012. Anti-angiogenic and anti-tumor activity of bavachinin by targeting hypoxia-inducible factor-1α. European Journal of Pharmacology. 691 (1–3): 28–37. https://doi.org/10.1016/j.ejphar.2012.06.028.
Pai, J.-T., M.W. Hsu, Y.L. Leu, et al. 2021. Induction of G2/M cell cycle arrest via p38/p21-dependent signaling pathway activation by bavachinin in non-small-cell lung cancer cells. Molecules 26 (17). https://doi.org/10.3390/molecules26175161.
Zhao, C., B. Ghosh, T. Chakraborty, et al. 2021. Bavachinin mitigates DMH induced colon cancer in rats by altering p53/Bcl2/BAX signaling associated with apoptosis. Biotechnic & histochemistry : Official publication of the Biological Stain Commission. 96 (3): 179–190. https://doi.org/10.1080/10520295.2020.1778087.
Chen, X., T. Wen, J. Wei, et al. 2013. Treatment of allergic inflammation and hyperresponsiveness by a simple compound, bavachinin, isolated from Chinese herbs. Cellular & Molecular Immunology. 10 (6): 497–505. https://doi.org/10.1038/cmi.2013.27.
Poornima, P., J.D. Kumar, Q. Zhao, et al. 2016. Network pharmacology of cancer: From understanding of complex interactomes to the design of multi-target specific therapeutics from nature. Pharmacological Research. 111: 290–302. https://doi.org/10.1016/j.phrs.2016.06.018.
Yang, S., J. Zhang, Y. Yan, et al. 2019. Network pharmacology-based strategy to investigate the pharmacologic mechanisms of Koidz. for the Treatment of Chronic Gastritis. Frontier Pharmacology 10, 1629. https://doi.org/10.3389/fphar.2019.01629.
Hopkins, A.L. 2008. Network pharmacology: The next paradigm in drug discovery. Nature Chemical Biology 4 (11): 682–690. https://doi.org/10.1038/nchembio.118.
Kim, S., J. Chen, T. Cheng, et al. 2021. PubChem in 2021: New data content and improved web interfaces. Nucleic Acids Research 49 (D1): D1388–D1395. https://doi.org/10.1093/nar/gkaa971.
Daina, A., O. Michielin, and V. Zoete. 2019. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Research 47 (W1): W357–W364. https://doi.org/10.1093/nar/gkz382.
Wang, X., Y. Shen, S. Wang, et al. 2017. PharmMapper 2017 update: A web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Research 45 (W1): W356–W360. https://doi.org/10.1093/nar/gkx374.
Rappaport, N., M. Twik, I. Plaschkes, et al. 2017. MalaCards: An amalgamated human disease compendium with diverse clinical and genetic annotation and structured search. Nucleic Acids Research 45 (D1): D877–D887. https://doi.org/10.1093/nar/gkw1012.
Wishart, D. S., Y.D. Feunang, A.C. Guo, et al. 2018. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Research 46 (D1): D1074–D1082. https://doi.org/10.1093/nar/gkx1037.
Zhou, Y., Y. Zhang, X. Lian, et al. 2022. Therapeutic target database update 2022: Facilitating drug discovery with enriched comparative data of targeted agents. Nucleic Acids Research 50 (D1): D1398–D1407. https://doi.org/10.1093/nar/gkab953.
Piñero, J., J.M. Ramírez-Anguita, J. Saüch-Pitarch, et al. 2020. The DisGeNET knowledge platform for disease genomics: 2019 update. Nucleic Acids Research 48 (D1): D845–D855. https://doi.org/10.1093/nar/gkz1021.
UniProt: the universal protein knowledgebase in 2021. 2021. Nucleic Acids Research 49 (D1): D480–D489. https://doi.org/10.1093/nar/gkaa1100.
Firestein, G.S. 2003. Evolving concepts of rheumatoid arthritis. Nature 423 (6937): 356–361. https://doi.org/10.1038/nature01661.
Bartok, B., and G.S. Firestein. 2010. Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunological Reviews 233 (1): 233–255. https://doi.org/10.1111/j.0105-2896.2009.00859.x.
Filer, A., L.S.C. Ward, S. Kemble, et al. 2017. Identification of a transitional fibroblast function in very early rheumatoid arthritis. Annals of the Rheumatic Diseases 76 (12): 2105–2112. https://doi.org/10.1136/annrheumdis-2017-211286.
Zhang, L.-M., J.J. Zhou and C.L. Luo. 2018. CYLD suppression enhances the pro-inflammatory effects and hyperproliferation of rheumatoid arthritis fibroblast-like synoviocytes by enhancing NF-κB activation. Arthritis Research and Therapy 20 (1): 219. https://doi.org/10.1186/s13075-018-1722-9.
Zhu, S.-L., J.-L. Huang, W.-X. Peng, et al. 2017. Inhibition of smoothened decreases proliferation of synoviocytes in rheumatoid arthritis. Cellular & Molecular Immunology. 14 (2): 214–222. https://doi.org/10.1038/cmi.2015.67.
Leverson, J. D., D.C. Phillips, M.J. Mitten, et al. 2015. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Science Translational Medicine 7 (279): 279ra40. https://doi.org/10.1126/scitranslmed.aaa4642.
Mahajan, S.D., V.M. Tutino, Y. Redae, et al. 2016. C5a induces caspase-dependent apoptosis in brain vascular endothelial cells in experimental lupus. Immunology 148 (4): 407–419. https://doi.org/10.1111/imm.12619.
Tsuruta, F., N. Masuyama, and Y. Gotoh. 2002. The phosphatidylinositol 3-kinase (PI3K)-Akt pathway suppresses Bax translocation to mitochondria. Journal of Biological Chemistry 277 (16): 14040–14047. https://doi.org/10.1074/jbc.M108975200.
Carneiro, B.A., and W.S. El-Deiry. 2020. Targeting apoptosis in cancer therapy. Nature Reviews. Clinical Oncology 17 (7): 395–417. https://doi.org/10.1038/s41571-020-0341-y.
Pickens, S.R., M.V. Volin, A.M. Mandelin, et al. 2010. IL-17 contributes to angiogenesis in rheumatoid arthritis. The Journal of Immunology 184 (6): 3233–3241. https://doi.org/10.4049/jimmunol.0903271.
Brennan, F.M., and I.B. McInnes. 2008. Evidence that cytokines play a role in rheumatoid arthritis. The Journal of Clinical Investigation 118 (11): 3537–3545. https://doi.org/10.1172/JCI36389.
Davignon, J.-L., B. Rauwel, Y. Degboé, et al. 2018. Modulation of T-cell responses by anti-tumor necrosis factor treatments in rheumatoid arthritis: a review. Arthritis Research and Therapy 20 (1): 229. https://doi.org/10.1186/s13075-018-1725-6.
Sur, B., S. Kang, M. Kim, et al. 2019. Inhibition of carrageenan/kaolin-induced arthritis in rats and of inflammatory cytokine expressions in human IL-1β-stimulated fibroblast-like synoviocytes by a benzylideneacetophenone derivative. Inflammation 42 (3): 928–936. https://doi.org/10.1007/s10753-018-0947-8.
Ruderman, E.M. 2015. Rheumatoid arthritis: IL-6 inhibition in RA–déjà vu all over again? Nature Reviews Rheumatology 11 (6): 321–322. https://doi.org/10.1038/nrrheum.2015.58.
Mitchell, J., S.J. Kim, G. Koukos, et al. 2018. Colonic inhibition of phosphatase and tensin homolog increases colitogenic bacteria, causing development of colitis in Il10-/- mice. Inflammatory Bowel Diseases 24 (8): 1718–1732. https://doi.org/10.1093/ibd/izy124.
Borges, G.R., D.A. Morgan, P. Ketsawatsomkron, et al. 2014. Interference with peroxisome proliferator-activated receptor-γ in vascular smooth muscle causes baroreflex impairment and autonomic dysfunction. Hypertension 64 (3): 590–596. https://doi.org/10.1161/HYPERTENSIONAHA.114.03553.
Pestereva, E., S. Kanakasabai, and J.J. Bright. 2012. PPARγ agonists regulate the expression of stemness and differentiation genes in brain tumour stem cells. British Journal of Cancer 106 (10): 1702–1712. https://doi.org/10.1038/bjc.2012.161.
Li, X.-F., Y.Y. Sun, J. Bao, et al. 2017. Functional role of PPAR-γ on the proliferation and migration of fibroblast-like synoviocytes in rheumatoid arthritis. Scientific Reports 7 (1): 12671. https://doi.org/10.1038/s41598-017-12570-6.
Li, X.-F., S.-Q. Yin, H. Li, et al. 2022. PPAR-γ alleviates the inflammatory response in TNF-α-induced fibroblast-like synoviocytes by binding to p53 in rheumatoid arthritis. Acta Pharmacologica Sinica. https://doi.org/10.1038/s41401-022-00957-9.
Yang, H., Y. Luo, and X. Lai. 2022. Il-34 regulates MAPKs, PI3K/Akt, JAK and NF-κB pathways and induces the expression of inflammatory factors in RA-FLS. Clinical and Experimental Rheumatology 40 (9): 1779–1788. https://doi.org/10.55563/clinexprheumatol/6t1d4i.
Li, X., and Y. Wang. 2020. Cinnamaldehyde attenuates the progression of rheumatoid arthritis through down-regulation of PI3K/AKT signaling pathway. Inflammation 43 (5): 1729–1741. https://doi.org/10.1007/s10753-020-01246-5.
Funding
This work was supported by grants from the Natural Science Foundation of China (31701104), the National Undergraduate Innovation and Entrepreneurship Program (202213705019), and the Chengdu Medical College Graduate Student Innovation Fund (YCX2022-03–04).
Author information
Authors and Affiliations
Contributions
Conceptualization, H.-D. and W.-K.S.; Investigation, H.-D.and J.S.; Methodology, H.-D.and L.-J.W.; Supervision, W.-K.S.; Visualization, H.-D., J.J., and L.-J.W.; Experimental validation, H.-D., J.J., and M.-H.; Writing—original draft, H.-D.; Writing—review and editing, W.-K.S., Q.-L.Z., and L.-J.W. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
The animal study was reviewed and approved by the Ethics Committee for Animal Experiments of Chengdu Medical College.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Deng, H., Jiang, J., Shu, J. et al. Bavachinin Ameliorates Rheumatoid Arthritis Inflammation via PPARG/PI3K/AKT Signaling Pathway. Inflammation 46, 1981–1996 (2023). https://doi.org/10.1007/s10753-023-01855-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-023-01855-w