Abstract
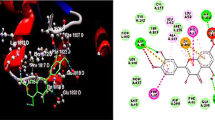
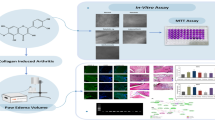
The effectiveness of curcumin in preventing and treating collagen-induced inflammatory arthritis (CIA) in rats and oxidative stress in rats was investigated. We investigated curcumin’s curative and preventive effects on paw edema, arthritic size, body weight, and radiologic and histological joint abnormalities. It has been shown that curcumin may dramatically lower the risk of developing arthritis. In addition, the number of white blood cells (WBCs) in the body has dropped, which is a strong indication that curcumin has anti-inflammatory characteristics. A follow-up theoretical investigation of curcumin molecular docking on xanthine oxidase (XO) was carried out after the properties of curcumin were determined using the conductor-like screening model for real solvents (COSMO-RS) theory. Because of the interaction between curcumin and the residues on XO named Ile264, Val259, Asn351, and Leu404, XO may be suppressed by this molecule. Curcumin’s anti-inflammatory and antioxidant properties may be responsible for the anti-arthritic effects that have been seen on oxidative stress markers and XO. On the other hand, more research is being conducted to understand its function better in the early stages of rheumatoid arthritis (RA). To determine whether or not curcumin interacts with AR targets, a molecular docking study was conducted using MVD software against TNFRSF11A and cathepsin L.












Similar content being viewed by others
References
Scherlinger, M., P. Mertz, F. Sagez, A. Meyer, R. Felten, E. Chatelus, R.-M. Javier, C. Sordet, T. Martin, A.-S. Korganow, A. Guffroy, V. Poindron, C. Richez, M.-E. Truchetet, P. Blanco, T. Schaeverbeke, J. Sibilia, H. Devillers, and L. Arnaud. 2020. Worldwide trends in all-cause mortality of auto-immune systemic diseases between 2001 and 2014. Autoimmunity Reviews 19 (6): 102531. https://doi.org/10.1016/j.autrev.2020.102531.
Burgos-Vargas, R., L.J. Catoggio, C. Galarza-Maldonado, K. Ostojich, and M.H. Cardiel. 2013. Current therapies in rheumatoid arthritis: A Latin American perspective. Reumatología Clínica 9 (2): 106–112. https://doi.org/10.1016/j.reuma.2012.09.001.
Paleolog, E.M. 2002. Angiogenesis in rheumatoid arthritis. Arthritis Research 4: S81-90. https://doi.org/10.1007/978-3-7091-1428-5_16.
Pincus, T., and L.F. Callahan. 1993. What is the natural history of rheumatoid arthritis? Rheumatic Diseases Clinics of North America 19 (1): 123–151. https://doi.org/10.1016/S0889-857X(21)00171-X.
Imboden, J.B. 2009. The immunopathogenesis of rheumatoid arthritis. Annual Review of Pathology: Mechanisms of Disease 4 (1): 417–434. https://doi.org/10.1146/annurev.pathol.4.110807.092254.
Dewar, C.L., and M. Harth. 1994. Superoxide production from cytokine-treated adherent rheumatoid neutrophils. Clinical and Investigative Medicine 17 (1): 52–60.
Hanachi, N., N. Charef, A. Baghiani, S. Khennouf, Y. Derradji, S. Boumerfeg, D. Harzallah, and L. Arrar. 2009. Comparison of xanthine oxidase levels in synovial fluid from patients with rheumatoid arthritis and other joint inflammations. Saudi Medical Journal 30 (11): 1422–1425.
Scott, D.L., M. Shipley, A. Dawson, S. Edwards, D.P. Symmons, and A.D. Woolf. 1998. The clinical management of rheumatoid arthritis and osteoarthritis: Strategies for improving clinical effectiveness. Rheumatology 37 (5): 546–554. https://doi.org/10.1093/rheumatology/37.5.546.
Rooney, B.K., and A.J. Silman. 1999. Epidemiology of the rheumatic diseases. Current Opinion in Rheumatology 11 (2): 91–97. https://doi.org/10.1097/00002281-199903000-00002.
Payne, R. 2000. Limitations of NSAIDs for pain management: Toxicity or lack of efficacy? The Journal of Pain 1 (3): 14–18. https://doi.org/10.1054/jpai.2000.16611.
Ramadan, G., M.A. Al-Kahtani, and W.M. El-Sayed. 2011. Anti-inflammatory and antioxidant properties of Curcuma longa (turmeric) versus Zingiber officinale (ginger) rhizomes in rat adjuvant-induced arthritis. Inflammation 34 (4): 291–301. https://doi.org/10.1007/s10753-010-9278-0.
Kremers, H.M., P. Nicola, C.S. Crowson, W.M. O’Fallon, and S.E. Gabriel. 2004. Therapeutic strategies in rheumatoid arthritis over a 40-year period. Journal of Rheumatology 31 (12): 2366–2373.
Goel, A., A.B. Kunnumakkara, and B.B. Aggarwal. 2008. Curcumin as “curecumin”: From kitchen to clinic. Biochemical Pharmacology 75 (4): 787–809. https://doi.org/10.1016/j.bcp.2007.08.016.
Strimpakos, A.S., and R.A. Sharma. 2008. Curcumin: Preventive and therapeutic properties in laboratory studies and clinical trials. Antioxidants & Redox Signaling 10 (3): 511–546. https://doi.org/10.1089/ars.2007.1769.
Khopde, S.M., K.I., Priyadarsini, P., Venkatesan, and M.N.A, Rao. 1999. Free radical scavenging ability and antioxidant efficiency of curcumin and its substituted analogue. Biophysical Chemistry 80(2): 85–91. https://doi.org/10.1016/S0301-4622(99)00070-8.
Gupta, S.C., S. Prasad, J.H. Kim, S. Patchva, L.J. Webb, I.K. Priyadarsini, and B.B. Aggarwal. 2011. Multitargeting by curcumin as revealed by molecular interaction studies. Natural Products Reports 28 (12): 1937. https://doi.org/10.1039/c1np00051a.
Aggarwal, B.B., and K.B. Harikumar. 2009. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. International Journal of Biochemistry & Cell Biology 41 (1): 40–59. https://doi.org/10.1016/j.biocel.2008.06.010.
Kannan, K., R.A. Ortmann, and D. Kimpel. 2005. Animal models of rheumatoid arthritis and their relevance to human disease. Pathophysiology 12 (3): 167–181. https://doi.org/10.1016/j.pathophys.2005.07.011.
Tong, B., X. Yuan, Y. Dou, X. Wu, Y. Wang, Y. Xia, and Y. Dai. 2016. Sinomenine induces the generation of intestinal Treg cells and attenuates arthritis via activation of aryl hydrocarbon receptor. Laboratory Investigation 96 (10): 1076–1086. https://doi.org/10.1038/labinvest.2016.86.
Du, F., L. Lü, Q. Fu, M. Dai, J. Teng, W. Fan, S. Chen, P. Ye, N. Shen, X. Huang, J. Qian, and C. Bao. 2008. T-614, a Novel immunomodulator, attenuates joint inflammation and articular damage in collagen-induced arthritis. Arthritis Research & Therapy 10 (6): R136. https://doi.org/10.1186/ar2554.
Shealy, D.J., P.H. Wooley, E. Emmell, A. Volk, A. Rosenberg, G. Treacy, C.L. Wagner, L. Mayton, D.E. Griswold, and X.-Y.R. Song. 2002. Anti-TNF-alpha antibody allows healing of joint damage in polyarthritic transgenic mice. Arthritis Research 4 (5): R7. https://doi.org/10.1186/ar430.
Zhu, L., W. Wei, Y.-Q. Zheng, and X.-Y. Jia. 2005. Effects and mechanisms of total glucosides of paeony on joint damage in rat collagen-induced arthritis. Inflammation Research 54 (5): 211–220. https://doi.org/10.1007/s00011-005-1345-x.
Bai, S., and B. Lu. 2014. VCMM: A visual tool for continuum molecular modeling. Journal of Molecular Graphics and Modelling 50: 44–49. https://doi.org/10.1016/j.jmgm.2014.03.006.
Ellman, G.L. 1959. Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics 82 (1): 70–77. https://doi.org/10.1016/0003-9861(59)90090-6.
Ohkawa, H., N. Ohishi, and K. Yagi. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry 95 (2): 351–358. https://doi.org/10.1016/0003-2697(79)90738-3.
Aebi, H. 1984. Catalase in vitro. pp 121–126. https://doi.org/10.1016/S0076-6879(84)05016-3.
Nandy, S., H.S. Paul, and N.R. Barman. 2012. In vitro evaluation of antioxidant activity of Leucas plukenetii ( Roth ) Spreng. Asian Journal of Plant Science and Research 2 (3): 254–262.
Zheng, L., and D. Dong. 2011. Development and validation of an HPLC method for simultaneous determination of nine active components in “Da-Chai-Hu-Tang.” Chinese Medicine 02 (01): 20–28. https://doi.org/10.4236/cm.2011.21004.
Gornall, A.G., C.J. Bardawill, and M.M. David. 1949. Determination of serum proteins by means of the biuret reaction. Journal of Biological Chemistry 177 (2): 751–766.
Song, Z., Q. Zeng, J. Zhang, H. Cheng, L. Chen, and Z. Qi. 2016. Solubility of imidazolium-based ionic liquids in model fuel hydrocarbons: A COSMO-RS and Experimental Study. Journal of Molecular Liquids 224: 544–550. https://doi.org/10.1016/j.molliq.2016.10.026.
Evers, F., F. Weigend, and M. Koentopp. 2003. Coherent transport through a molecular wire: DFT calculation. Phys. E Low-dimensional Syst. Nanostructures 18 (1–3): 255–257. https://doi.org/10.1016/S1386-9477(02)01006-8.
Delley, B. 1990. An all-electron numerical method for solving the local density functional for polyatomic molecules. The Journal of Chemical Physics 92 (1): 508–517. https://doi.org/10.1063/1.458452.
Kanouni, K.E., Y. Benguerba, and A. Erto. 2019. Theoretical investigation of the solubility of some antiemetic drugs. Journal of Molecular Liquids 282: 626–632. https://doi.org/10.1016/j.molliq.2019.03.028.
Alioui, O., Y. Benguerba, and I.M. Alnashef. 2020. Investigation of the CO2-solubility in deep eutectic solvents using COSMO-RS and molecular dynamics methods. Journal of Molecular Liquids 307: 113005. https://doi.org/10.1016/j.molliq.2020.113005.
Benabid, S., N. Haddaoui, T. Lemaoui, A.S. Darwish, Y. Benguerba, and I.M. Alnashef. 2021. Computational modeling of polydecanediol-co-citrate using benzalkonium chloride-based hydrophobic eutectic solvents: COSMO-RS, reactivity, and compatibility insights. Journal of Molecular Liquids 339: 116674. https://doi.org/10.1016/j.molliq.2021.116674.
Bououden, W., Y. Benguerba, A.S. Darwish, A. Attoui, T. Lemaoui, M. Balsamo, A. Erto, and I.M. Alnashef. 2021. Surface adsorption of crizotinib on carbon and boron nitride nanotubes as anti-cancer drug carriers: COSMO-RS and DFT molecular insights. Journal of Molecular Liquids 338: 116666. https://doi.org/10.1016/j.molliq.2021.116666.
Benabid, S., Y. Benguerba, I.M. AlNashef, and N. Haddaoui. 2019. Theoretical study of physicochemical properties of selected ammonium salt-based deep eutectic solvents. Journal of Molecular Liquids 285: 38–46. https://doi.org/10.1016/j.molliq.2019.04.052.
Pérez, P., L.R., Domingo, M., Duque-Noreña, and E.A., Chamorro. 2009. condensed-to-atom nucleophilicity index. An application to the director effects on the electrophilic aromatic substitutions. Journal of Molecular Structure: THEOCHEM 895(1–3): 86–91. https://doi.org/10.1016/j.theochem.2008.10.014.
Parr, R.G., and W. Yang. 1984. Density functional approach to the frontier-electron theory of chemical reactivity. Journal of the American Chemical Society 106 (14): 4049–4050. https://doi.org/10.1021/ja00326a036.
Tarek, L., H.N. El Houda, B. Yacine, and A. Ayoub. 2019. Molecular docking of new active compounds towards the acetylcholinesterase enzyme. Current Research Bioinformatics 8 (1): 18–20. https://doi.org/10.3844/ajbsp.2019.18.20.
Kanouni, K.E., and Y. Benguerba. 2020. Theoretical investigation of two antiemetic drugs at DFT level. Current Research Bioinformatics 9 (1): 17–25. https://doi.org/10.3844/ajbsp.2020.17.25.
Lemaoui, T., A.S. Darwish, N.E.H. Hammoudi, F. Abu Hatab, A. Attoui, I.M. Alnashef, and Y. Benguerba. 2020. Prediction of electrical conductivity of deep eutectic solvents using COSMO-RS sigma profiles as molecular descriptors: A quantitative structure-property relationship study. Industrial and Engineering Chemistry Research 59 (29): 13343–13354. https://doi.org/10.1021/acs.iecr.0c02542.
Lemaoui, T., N.E.H. Hammoudi, I.M. Alnashef, M. Balsamo, A. Erto, B. Ernst, and Y. Benguerba. 2020. Quantitative structure properties relationship for deep eutectic solvents using Sσ-profile as molecular descriptors. Journal of Molecular Liquids 309: 113165. https://doi.org/10.1016/j.molliq.2020.113165.
Lemaoui, T., A.S. Darwish, A. Attoui, F. Abu Hatab, N.E.H. Hammoudi, Y. Benguerba, L.F. Vega, and I.M. Alnashef. 2020. Predicting the density and viscosity of hydrophobic eutectic solvents: Towards the development of sustainable solvents. Green Chemistry 22 (23): 8511–8530. https://doi.org/10.1039/d0gc03077e.
Lemaoui, T., F. Abu Hatab, A.S. Darwish, A. Attoui, N.E.H. Hammoudi, G. Almustafa, M. Benaicha, Y. Benguerba, and I.M. Alnashef. 2021. Molecular-based guide to predict the pH of eutectic solvents: Promoting an efficient design approach for new green solvents. ACS Sustainable Chemistry & Engineering 9 (17): 5783–5808. https://doi.org/10.1021/acssuschemeng.0c07367.
Darwish, A.S., F., Abu Hatab, T., Lemaoui, O.A.Z., Ibrahim, G, Almustafa, B., Zhuman, S.E.E., Warrag, M.K., Hadj-Kali, Y., Benguerba, and I.M., Alnashef. 2021. Multicomponent extraction of aromatics and heteroaromatics from diesel using acidic eutectic solvents: experimental and COSMO-RS predictions. Journal of Molecular Liquid 336: 116575. https://doi.org/10.1016/j.molliq.2021.116575.
McInnes, I.B., and G. Schett. 2011. The pathogenesis of rheumatoid arthritis. New England Journal of Medicine 365 (23): 2205–2219. https://doi.org/10.1056/NEJMra1004965.
García-González, A., R., Gaxiola-Robles, and T., Zenteno-Savín. 2015. Oxidative stress in patients with rheumatoid arthritis. Revista de Investigacion Clinica 67 (1): 46–53.
Sobhi, W. 2020. Involvement of oxidative stress in type 1 diabetes. American Journal of Biomedical Science Research 6 (6): 538–543. https://doi.org/10.34297/ajbsr.2020.06.001100.
Stevens, C.R., M. Benboubetra, R. Harrison, T. Sahinoglu, E.C. Smith, and D.R. Blake. 1991. Localisation of xanthine oxidase to synovial endothelium. Annals of the Rheumatic Diseases 50 (11): 760–762. https://doi.org/10.1136/ard.50.11.760.
Henrotin, Y., A.L. Clutterbuck, D. Allaway, E.M. Lodwig, P. Harris, M. Mathy-Hartert, M. Shakibaei, and A. Mobasheri. 2010. Biological actions of curcumin on articular chondrocytes. Osteoarthritis and Cartilage 18 (2): 141–149. https://doi.org/10.1016/j.joca.2009.10.002.
Banerjee, M., L.M. Tripathi, V.M.L. Srivastava, A. Puri, and R. Shukla. 2003. Modulation of inflammatory mediators by ibuprofen and curcumin treatment during chronic inflammation in rat. Immunopharmacology and Immunotoxicology 25 (2): 213–224. https://doi.org/10.1081/IPH-120020471.
Anand, P., A.B. Kunnumakkara, R.A. Newman, and B.B. Aggarwal. 2007. Bioavailability of Curcumin: Problems and Promises. Molecular Pharmaceutics 4 (6): 807–818. https://doi.org/10.1021/mp700113r.
Bisset, S., W. Sobhi, C. Bensouici, and A. Khenchouche. 2020. Chain-Breaking/Preventive Antioxidant, Urate-Lowering, and Anti-Inflammatory Effects of Pure Curcumin. Current Nutrition & Food Science 17 (1): 66–74. https://doi.org/10.2174/1573401316999200421095134.
Naik, S.R., V.N. Thakare, and S.R. Patil. 2011. Protective effect of curcumin on experimentally induced inflammation, hepatotoxicity and cardiotoxicity in rats: Evidence of its antioxidant property. Experimental and Toxicologic Pathology 63 (5): 419–431. https://doi.org/10.1016/j.etp.2010.03.001.
Srivastava, R.M., S. Singh, S.K. Dubey, K. Misra, and A. Khar. 2011. Immunomodulatory and therapeutic activity of curcumin. International Immunopharmacology 11 (3): 331–341. https://doi.org/10.1016/j.intimp.2010.08.014.
Bresnihan, B., J.M. Alvaro-Gracia, M. Cobby, M. Doherty, Z. Domljan, P. Emery, G. Nuki, K. Pavelka, R. Rau, B. Rozman, I. Watt, B. Williams, R. Aitchison, D. McCabe, and P. Musikic. 1998. Treatment of rheumatoid arthritis with recombinant human interleukin-1 receptor antagonist. Arthritis and Rheumatism 41 (12): 2196–2204. https://doi.org/10.1002/1529-0131(199812)41:12%3c2196::AID-ART15%3e3.0.CO;2-2.
Smeets, T.J.M., M.C. Kraan, M.E. van Loon, and P.-P. Tak. 2003. Tumor necrosis factor ? Blockade reduces the synovial cell infiltrate early after initiation of treatment, but apparently not by induction of apoptosis in synovial tissue. Arthritis and Rheumatism 48 (8): 2155–2162. https://doi.org/10.1002/art.11098.
Ranjan, D., C. Chen, T.D. Johnston, H. Jeon, and M. Nagabhushan. 2004. Curcumin Inhibits Mitogen Stimulated lymphocyte proliferation, NFκB activation, and IL-2 signaling. Journal of Surgical Research 121 (2): 171–177. https://doi.org/10.1016/j.jss.2004.04.004.
Abe, Y., S. Hashimoto, and T. Horie. 1999. Curcumin inhibition of inflammation cytokine production by human peripheral blood monocytes and alveolar macrophages. Pharmacological Research 39 (1): 41–47. https://doi.org/10.1006/phrs.1998.0404.
Epstein, F.H., and E.D. Harris. 1990. Rheumatoid arthritis: Pathophysiology and implications for therapy. New England Journal of Medicine 322 (18): 1277–1289. https://doi.org/10.1056/NEJM199005033221805.
Teitelbaum, S.L. 2000. Bone resorption by osteoclasts. Science (80-.) 289(5484): 1504–1508. https://doi.org/10.1126/science.289.5484.1504.
Redlich, K., E.F., Wagner, G., Schett, K., Redlich, S., Hayer, R., Ricci, J., David, M., Tohidast-akrad, G., Kollias, G., Steiner, J.S., Smolen, E.F., Wagner, and G., Schett. 2002 Osteoclasts are essential for TNF- a – mediated joint destruction Find the Latest Version : Joint Destruction 110(10): 1419–1427. https://doi.org/10.1172/JCI200215582.Introduction.
Zahidah, A.F., O. Faizah, K. Nur Aqilah, and K. Anna Taty. 2012. Curcumin as an anti-arthritic agent in collagen-induced arthritic Sprague-Dawley rats. Sains Malaysiana 41 (5): 591–595.
Youssef, P.P., T.J. Smeets, B. Bresnihan, G. Cunnane, O. Fitzgerald, F. Breedveld, and P.P. Tak. 1998. Microscopic Measurement of cellular infiltration in the rheumatoid arthritis synovial membrane: A comparison of semiquantitative and quantitative analysis. British Journal of Rheumatology 37 (9): 1003–1007. https://doi.org/10.1093/rheumatology/37.9.1003.
Abou-Seif, M.A., and A.-A. Youssef. 2004. Evaluation of some biochemical changes in diabetic patients. Clinica Chimica Acta 346 (2): 161–170. https://doi.org/10.1016/j.cccn.2004.03.030.
Olayinka, E., A. Ore, O. Adeyemo, O. Ola, O. Olotu, and R. Echebiri. 2015. Quercetin, a Flavonoid antioxidant, ameliorated procarbazine-induced oxidative damage to murine tissues. Antioxidants 4 (2): 304–321. https://doi.org/10.3390/antiox4020304.
Gottschalk, T.A., E., Tsantikos, and M.L., Hibbs. 2015. Pathogenic inflammation and its therapeutic targeting in systemic lupus erythematosus. Frontier in Immunology. 6. https://doi.org/10.3389/fimmu.2015.00550.
Kalpana, C., and V.P., Menon. 2004. Modulatory effects of curcumin on lipid peroxidation and antioxidant status during nicotine-induced toxicity. Polish Journal of Pharmacology 56(5): 581–586.
Menon, V.P., and A.R, Sudheer. 2007. Antioxidant and anti-inflammatory properties of curcumin. in The Molecular Targets and Therapeutic Uses of Curcumin in Health and Disease; Springer US: Boston, MA; pp 105–125. https://doi.org/10.1007/978-0-387-46401-5_3.
Samarghandian, S., M. Azimi-Nezhad, T. Farkhondeh, and F. Samini. 2017. Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomedicine & Pharmacotherapy 87: 223–229. https://doi.org/10.1016/j.biopha.2016.12.105.
Momeni, H.R., and N. Eskandari. 2017. Effect of curcumin on kidney histopathological changes, lipid peroxidation and total antioxidant capacity of serum in sodium arsenite-treated mice. Experimental and Toxicologic Pathology 69 (2): 93–97. https://doi.org/10.1016/j.etp.2016.08.006.
Boudiaf, K., Z. Houcher, W. Sobhi, and M. Benboubetra. 2010. Evaluation of antioxidant and anti-xanthine oxidoreductase activities of Nigella sativa Linn seeds’ extracts. Journal of Applied Biological Sciences 4 (1): 7–16.
Khither, H., S., Madoui, K., Mokhnache, and W., Sobhi. 2020. Evaluation of in vitro and in vivo anti-arthritic and xanthine oxidase inhibitory activities of thymoquinone: applied to collagen-induced rheumatoid arthritis in male rats. No. November.
Allen, R.E., J.M. Outhwaite, C.J. Morris, and D.R. Blake. 1987. Xanthine oxidoreductase is present in human synovium. Annals of the Rheumatic Diseases 46 (11): 843–845. https://doi.org/10.1136/ard.46.11.843.
Miesel, R., and M. Zuber. 1993. Elevated levels of xanthine oxidase in serum of patients with inflammatory and autoimmune rheumatic diseases. Inflammation 17 (5): 551–561. https://doi.org/10.1007/BF00914193.
Sarmiento, C., A., Chiou, M.J., Duryee, J., Tian, G.M., Thiele, D.R., Anderson, T.R., Mikuls, and M.C., Zimmerman. 2017. Methotrexate scavenges xanthine oxidase-derived superoxide without inhibiting xanthine oxidase activity. The FASEB Journal 31(S1): lb689–lb689. https://doi.org/10.1096/fasebj.31.1_supplement.lb689.
Hughes, A.E., S.H. Ralston, J. Marken, C. Bell, H. MacPherson, R.G. Wallace, W. van Hul, M.P. Whyte, K. Nakatsuka, L. Hovy, and D.M. Anderson. 2000. Mutations in TNFRSF11A, affecting the signal peptide of RANK, cause familial expansile osteolysis. Nature Genetics 24 (1): 45–48. https://doi.org/10.1038/71667.
Adán, N. J., Guzmán-Morales, M.G., Ledesma-Colunga, S.I., Perales-Canales, A., Quintanar-Stéphano, F., López-Barrera, I., Méndez, B., Moreno-Carranza, J., Triebel, N., Binart, G., Martínez de la Escalera, S., Thebault, and C., Clapp. 2013. Prolactin promotes cartilage survival and attenuates inflammation in inflammatory arthritis. The Journal of Clinical Investigation 123(9): 3902–3913. https://doi.org/10.1172/JCI69485.
Ledesma-Colunga, M.G., N., Adán, G., Ortiz, M., Solís-Gutiérrez, F., López-Barrera, G., Martínez de la Escalera, and C., Clapp. 2017. Prolactin Blocks the expression of receptor activator of nuclear factor ΚB ligand and reduces osteoclastogenesis and bone loss in murine inflammatory arthritis. Arthritis Research & Therapy. 19(1): 93. https://doi.org/10.1186/s13075-017-1290-4.
Vasiljeva, O., T. Reinheckel, C. Peters, D. Turk, V. Turk, and B. Turk. 2007. Emerging roles of cysteine cathepsins in disease and their potential as drug targets. Current Pharmaceutical Design 13 (4): 387–403. https://doi.org/10.2174/138161207780162962.
Bethel, P.A., S. Gerhardt, E.V. Jones, P.W. Kenny, G.I. Karoutchi, A.D. Morley, K. Oldham, N. Rankine, M. Augustin, S. Krapp, H. Simader, and S. Steinbacher. 2009. Design of selective cathepsin inhibitors. Bioorganic & Medicinal Chemistry Letters 19 (16): 4622–4625. https://doi.org/10.1016/j.bmcl.2009.06.090.
Acknowledgements
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2022R457), King Saud University, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Contributions
Seghira Bisset, Widad Sobhi, Rezgui, Siham Ferdjioui, Yacine Derradji, and Abdelhalim Khenchouche contributed to the study conception and design, material preparation, data collection, and analysis. Conceptualization, supervision, and data analysis were performed by Seghira Bisset and Widad Sobhi. The docking study was done by Ayoub Attoui and Khalil Errahmane Kanouni. The first draft of the manuscript was written by all authors. Tarek Lamaoui, Manawwer Alam, and Yacine Benguerba contributed to the writing and editing of the article. All authors read and approved the final manuscript. Yousef A. Bin Jardan actively contributed to the editing and response to the reviewers’ comments. Shobhan Das contributed to improving the English and helped to respond to reviewers’ comments.
Corresponding authors
Ethics declarations
Ethics Approval
The animal experimental protocol reported in the study was approved by the Institutional Animal Ethics Committee (IAEC) of the University Ferhat Abbas Setif 1, Setif, Algeria.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bisset, S., Sobhi, W., Attoui, A. et al. Targeting Oxidative Stress Markers, Xanthine Oxidase, TNFRSF11A and Cathepsin L in Curcumin-Treated Collagen-Induced Arthritis: A Physiological and COSMO-RS Study. Inflammation 46, 432–452 (2023). https://doi.org/10.1007/s10753-022-01745-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-022-01745-7