Abstract
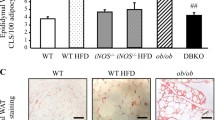
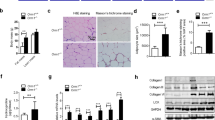
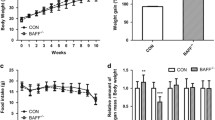
The E2 promoter binding factor 1 (E2F1) and the Wnt/β-catenin signaling are crucial in regulating metabolic homeostasis including obesity. The β-catenin interacting protein 1 (CTNNBIP1), also known as the inhibitor of β-catenin and TCF4 (ICAT), is required for E2F1 to inhibit the activity of β-catenin. However, the role of ICAT in E2F1 regulating obesity-related metabolic disorders remains unknown. In the present study, male adipose tissue-specific ICAT knockout (ICATadi−/−) C57BL/6 J mice and control littermates aged 6–8 weeks were fed with high-fat diet (HFD) for 12 weeks to explore the effect of ICAT on lipid metabolism and obesity-related disorders. Results showed that the adipose tissue-specific ICAT knockout had negligible effect on lipid metabolism, reflected by no difference in body weight, fat mass, and the expression of proteins involved in lipid metabolism in white adipose tissue (WAT) and the liver between the ICATadi−/− mice and the control littermate (ICATfl/fl) mice. However, the knockout of ICAT reduced inflammatory response in WAT and the liver. Additionally, Sirius red staining results showed that deletion of ICAT attenuated fibrosis and reduced mRNA levels of transforming growth factor β1(TGF-β1), matrix metallopeptidase 2 (Mmp2), Mmp3, and collagen, type V, alpha 1 (Col5a1) in WAT and the liver. These results suggested that knockout of ICAT improved the metabolic abnormalities of obese mice through attenuating adipose tissue and the liver inflammation as well as fibrosis. Our findings may provide a new insight to understand the role of ICAT in inflammation and fibrosis.






Similar content being viewed by others
Data Availability
Data that support the results of the present study are available from the corresponding author upon reasonable request.
Abbreviations
- eWAT:
-
Epididymal WAT
- iWAT:
-
Inguinal WAT
- ACC:
-
Acetyl-CoA carboxylase
- C/EBPα:
-
CAAT/enhancer binding protein α
- Col5a1 :
-
Collagen, type V, alpha 1
- E2F1:
-
E2 promoter binding factor 1
- ICAT:
-
β-Catenin interacting protein 1 (CTNNBIP1), inhibitor of β-catenin and TCF4
- Mmp2 :
-
Matrix metallopeptidase 2
- PPARγ:
-
Peroxisome proliferators-activated receptor γ
- TGF-β1 :
-
Transforming growth factor β1
- Timp2 (3) :
-
Tissue inhibitor of metalloproteinase 2 (3)
- WAT:
-
White adipose tissue
References
Cinti, S. 2018. Adipose organ development and remodeling. Comprehensive Physiology 8 (4): 1357–1431.
Backdahl, J., L. Franzen, L. Massier, Q. Li, J. Jalkanen, H. Gao, et al. 2021. Spatial mapping reveals human adipocyte subpopulations with distinct sensitivities to insulin. Cell Metabolism 33 (9): 1869–1882.
Rosen, E.D., and B.M. Spiegelman. 2014. What we talk about when we talk about fat. Cell 156 (1–2): 20–44.
Worldwide trends in body-mass index, and underweight, overweight, and obesity from. 1975. to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 390 (10113): 2627–2642.
Hogan, P., T. Dall, and P. Nikolov. 2003. Economic costs of diabetes in the US in 2002. Diabetes Care 26 (3): 917–932.
Flier, J.S. 2004. Obesity wars: Molecular progress confronts an expanding epidemic. Cell 116 (2): 337–350.
Ellulu, M.S., I. Patimah, H. Khaza’ai, A. Rahmat, and Y. Abed. 2017. Obesity and inflammation: The linking mechanism and the complications. Archives of Medical Science 13 (4): 851–863.
Xu, L., N. Nagata, and T. Ota. 2018. Glucoraphanin: A broccoli sprout extract that ameliorates obesity-induced inflammation and insulin resistance. Adipocyte 7 (3): 218–225.
Tang, Q.Q., and M.D. Lane. 2012. Adipogenesis: From stem cell to adipocyte. Annual Review of Biochemistry 81: 715–736.
Cawthorn, W.P., F. Heyd, K. Hegyi, and J.K. Sethi. 2007. Tumour necrosis factor-alpha inhibits adipogenesis via a beta-catenin/TCF4(TCF7L2)-dependent pathway. Cell Death and Differentiation 14 (7): 1361–1373.
Chen, M., P. Lu, Q. Ma, Y. Cao, N. Chen, W. Li, et al. 2020. CTNNB1/β-catenin dysfunction contributes to adiposity by regulating the cross-talk of mature adipocytes and preadipocytes. Science Advance 6(2): eaax9605.
Christodoulides, C., A. Scarda, M. Granzotto, G. Milan, E. Dalla Nora, J. Keogh, et al. 2006. WNT10B mutations in human obesity. Diabetologia 49 (4): 678–684.
Mattei, J., Q. Qi, F.B. Hu, F.M. Sacks, and L. Qi. 2012. TCF7L2 genetic variants modulate the effect of dietary fat intake on changes in body composition during a weight-loss intervention. American Journal of Clinical Nutrition 96 (5): 1129–1136.
Bowers, R.R., and M.D. Lane. 2008. Wnt signaling and adipocyte lineage commitment. Cell Cycle 7 (9): 1191–1196.
Rosen, E.D., and O.A. MacDougald. 2006. Adipocyte differentiation from the inside out. Nature Reviews: Molecular Cell Biology 7 (12): 885–896.
Haim, Y., M. Bluher, N. Slutsky, N. Goldstein, N. Kloting, I. Harman-Boehm, et al. 2015. Elevated autophagy gene expression in adipose tissue of obese humans: A potential non-cell-cycle-dependent function of E2F1. Autophagy 11 (11): 2074–2088.
Julien, C., B. Le-Bail, K. Ouazzani Touhami, N. Frulio, J.F. Blanc, J.P. Adam, et al. 2021. Hepatocellular adenoma rrisk factors of hemorrhage: Size is not the only concern!: Single-center retrospective experience of 261 patients. Annals of Surgery 274 (5): 843–850.
Luke, J.J., R. Bao, R.F. Sweis, S. Spranger, and T.F. Gajewski. 2019. WNT/β-catenin pathway activation correlates with immune exclusion across human cancers. Clinical Cancer Research 25 (10): 3074–3083.
Mandigo, A.C., W. Yuan, K. Xu, P. Gallagher, A. Pang, Y.F. Guan, et al. 2021. RB/E2F1 as a master regulator of cancer cell metabolism in advanced disease. Cancer Discovery 11 (9): 2334–2353.
Chun, J.N., M. Cho, S. Park, I. So, and J.H. Jeon. 2020. The conflicting role of E2F1 in prostate cancer: A matter of cell context or interpretational flexibility? Biochimical et Biophysica Acta-Review on Cancer 1873 (1): 188336.
Morris, E.J., J.Y. Ji, F. Yang, L. Di Stefano, A. Herr, N.S. Moon, et al. 2008. E2F1 represses beta-catenin transcription and is antagonized by both pRB and CDK8. Nature 455 (7212): 552–556.
Zhao, J., R. Ramos, and M. Demma. 2013. CDK8 regulates E2F1 transcriptional activity through S375 phosphorylation. Oncogene 32 (30): 3520–3530.
Wu, Z., S. Zheng, Z. Li, J. Tan, and Q. Yu. 2011. E2F1 suppresses Wnt/beta-catenin activity through transactivation of beta-catenin interacting protein ICAT. Oncogene 30 (37): 3979–3984.
Wells, J., C.R. Graveel, S.M. Bartley, S.J. Madore, and P.J. Farnham. 2002. The identification of E2F1-specific target genes. Proceedings of the National Academy of Sciences of the United States of America 99 (6): 3890–3895.
Annicotte, J.S., E. Blanchet, C. Chavey, I. Iankova, S. Costes, S. Assou, et al. 2009. The CDK4-pRB-E2F1 pathway controls insulin secretion. Nature Cell Biology 11 (8): 1017–1023.
Blanchet, E., J.S. Annicotte, S. Lagarrigue, V. Aguilar, C. Clape, C. Chavey, et al. 2011. E2F transcription factor-1 regulates oxidative metabolism. Nature Cell Biology 13 (9): 1146–1152.
Denechaud, P.D., I.C. Lopez-Mejia, A. Giralt, Q. Lai, E. Blanchet, B. Delacuisine, et al. 2016. E2F1 mediates sustained lipogenesis and contributes to hepatic steatosis. Journal of Clinical Investigation 126 (1): 137–150.
Giralt, A., P.D. Denechaud, I.C. Lopez-Mejia, B. Delacuisine, E. Blanchet, C. Bonner, et al. 2018. E2F1 promotes hepatic gluconeogenesis and contributes to hyperglycemia during diabetes. Molecular Metabolism 11: 104–112.
Lai, Q., A. Giralt, C. Le May, L. Zhang, B. Cariou, P. D. Denechaud, et al. 2017. E2F1 inhibits circulating cholesterol clearance by regulating Pcsk9 expression in the liver. Junior Chamber International Insight 2(10).
Lu, Z., G. Marcelin, F. Bauzon, H. Wang, H. Fu, S.L. Dun, et al. 2013. pRb is an obesity suppressor in hypothalamus and high-fat diet inhibits pRb in this location. The EMBO Journal 32 (6): 844–857.
Xiong, M., W. Hu, Y. Tan, H. Yu, Q. Zhang, C. Zhao, et al. 2021. Transcription factor E2F1 knockout promotes mice white adipose tissue browning through autophagy inhibition. Frontiers in Physiology 12: 748040.
Chen, J., Y. Yang, S. Li, Y. Yang, Z. Dai, F. Wang, et al. 2020. E2F1 regulates adipocyte differentiation and adipogenesis by activating ICAT. Cells 9(4): 1024.
Satoh, K., M. Kasai, T. Ishidao, K. Tago, S. Ohwada, Y. Hasegawa, et al. 2004. Anteriorization of neural fate by inhibitor of beta-catenin and T cell factor (ICAT), a negative regulator of Wnt signaling. Proceedings of the National Academy of Sciences of the United States of America 101 (21): 8017–8021.
Jia, H., N. Liu, Y. Zhang, C. Wang, Y. Yang, and Z. Wu. 2021. 3-Acetyldeoxynivalenol induces cell death through endoplasmic reticulum stress in mouse liver. Environmental Pollution 286: 117238.
Kent, L.N., and G. Leone. 2019. The broken cycle: E2F dysfunction in cancer. Nature Reviews: Cancer 19 (6): 326–338.
Fajas, L., R.L. Landsberg, Y. Huss-Garcia, C. Sardet, J.A. Lees, and J. Auwerx. 2002. E2Fs regulate adipocyte differentiation. Developmental Cell 3 (1): 39–49.
Graham, T.A., W.K. Clements, D. Kimelman, and W. Xu. 2002. The crystal structure of the beta-catenin/ICAT complex reveals the inhibitory mechanism of ICAT. Molecular Cell 10 (3): 563–571.
Kim, S.E., H. Huang, M. Zhao, X. Zhang, A. Zhang, M.V. Semonov, et al. 2013. Wnt stabilization of β-catenin reveals principles for morphogen receptor-scaffold assemblies. Science 340 (6134): 867–870.
Ji, L., B. Lu, Z. Wang, Z. Yang, J. Reece-Hoyes, C. Russ, et al. 2018. Identification of ICAT as an APC inhibitor, revealing Wnt-dependent inhibition of APC-Axin interaction. Molecular Cell 72(1): 37–47 e34.
Xu, H., G.T. Barnes, Q. Yang, G. Tan, D. Yang, C.J. Chou, et al. 2003. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. Journal of Clinical Investigation 112 (12): 1821–1830.
Weisberg, S.P., D. McCann, M. Desai, M. Rosenbaum, R.L. Leibel, and A.W. Ferrante Jr. 2003. Obesity is associated with macrophage accumulation in adipose tissue. Journal of Clinical Investigation 112 (12): 1796–1808.
Skurk, T., C. Alberti-Huber, C. Herder, and H. Hauner. 2007. Relationship between adipocyte size and adipokine expression and secretion. Journal of Clinical Endocrinology and Metabolism 92 (3): 1023–1033.
Lee, Y.S., J. Wollam, and J.M. Olefsky. 2018. An integrated view of immunometabolism. Cell 172 (1–2): 22–40.
Ebke, L.A., A.L. Nestor-Kalinoski, B.D. Slotterbeck, A.G. Al-Dieri, S. Ghosh-Lester, L. Russo, et al. 2014. Tight association between macrophages and adipocytes in obesity: Implications for adipocyte preparation. Obesity (Silver Spring) 22 (5): 1246–1255.
Lim, C.A., F. Yao, J.J. Wong, J. George, H. Xu, K.P. Chiu, et al. 2007. Genome-wide mapping of RELA(p65) binding identifies E2F1 as a transcriptional activator recruited by NF-kappaB upon TLR4 activation. Molecular Cell 27 (4): 622–635.
Warg, L.A., J.L. Oakes, R. Burton, A.J. Neidermyer, H.R. Rutledge, S. Groshong, et al. 2012. The role of the E2F1 transcription factor in the innate immune response to systemic LPS. American Journal of Physiology: Lung Cellular and Molecular Physiology 303 (5): L391-400.
Yang, I.V., S. Alper, B. Lackford, H. Rutledge, L.A. Warg, L.H. Burch, et al. 2011. Novel regulators of the systemic response to lipopolysaccharide. American Journal of Respiratory Cell and Molecular Biology 45 (2): 393–402.
Marcelin, G., A.L.M. Silveira, L.B. Martins, A.V. Ferreira, and K. Clement. 2019. Deciphering the cellular interplays underlying obesity-induced adipose tissue fibrosis. Journal of Clinical Investigation 129 (10): 4032–4040.
Walton, K.L., K.E. Johnson, and C.A. Harrison. 2017. Targeting TGF-β mediated SMAD signaling for the prevention of fibrosis. Frontiers in Pharmacology 8: 461.
Bonnans, C., J. Chou, and Z. Werb. 2014. Remodelling the extracellular matrix in development and disease. Nature Reviews: Molecular Cell Biology 15 (12): 786–801.
Reed, N.I., H. Jo, C. Chen, K. Tsujino, T.D. Arnold, W.F. DeGrado, et al. 2015. The αvβ1 integrin plays a critical in vivo role in tissue fibrosis. Science Translational Medicine 7(288): ra79.
Jernås, M., J. Palming, K. Sjöholm, E. Jennische, P.A. Svensson, B.G. Gabrielsson, et al. 2006. Separation of human adipocytes by size: Hypertrophic fat cells display distinct gene expression. FASEB Journal 20 (9): 1540–1542.
Chun, T.H., K.B. Hotary, F. Sabeh, A.R. Saltiel, E.D. Allen, and S.J. Weiss. 2006. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell 125 (3): 577–591.
Kim, J.Y., E. van de Wall, M. Laplante, A. Azzara, M.E. Trujillo, S.M. Hofmann, et al. 2007. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. Journal of Clinical Investigation 117 (9): 2621–2637.
Pellegrinelli, V., J. Heuvingh, O. du Roure, C. Rouault, A. Devulder, C. Klein, et al. 2014. Human adipocyte function is impacted by mechanical cues. Journal of Pathology 233 (2): 183–195.
Atzori, L., G. Poli, and A. Perra. 2009. Hepatic stellate cell: A star cell in the liver. International Journal of Biochemistry and Cell Biology 41 (8–9): 1639–1642.
Sano, A., E. Kakazu, S. Hamada, J. Inoue, M. Ninomiya, T. Iwata, et al. 2021. Steatotic hepatocytes release mature VLDL through methionine and tyrosine metabolism in a keap1-Nrf2-dependent manner. Hepatology 74 (3): 1271–1286.
Funding
This work was supported by the National Natural Science Foundation of China (No. 31625025 and No. 32000082), China Postdoctoral Science Foundation (No.2022M713405), and the 2115 Talent Development of China Agricultual University.
Author information
Authors and Affiliations
Contributions
All authors participated in the study and supported the publication. Zhenlong Wu designed and conducted the research. Material preparation, data collection, and analysis were performed by Zhuan Song, Ning Liu, Yu He, Jingqing Chen, and Jun Li. Fengchao Wang constructed the knockout mice. The draft of the manuscript was written by Zhuan Song.
Corresponding author
Ethics declarations
Ethics Approval
The experimental protocol was approved by the Protocol Management and Review Committee of China Agricultural University (Beijing, China). All mice were raised and conformed to the Institutional Animal Care and Use Committee of China Agricultural University (AW72602202-1–7).
Consent to Participate and Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Song, Z., Liu, N., He, Y. et al. Knockout of ICAT in Adipose Tissue Alleviates Fibro-inflammation in Obese Mice. Inflammation 46, 404–417 (2023). https://doi.org/10.1007/s10753-022-01742-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-022-01742-w